-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 1181-1183
doi:10.5923/j.ajmms.20251504.66
Received: Mar. 20, 2025; Accepted: Apr. 19, 2025; Published: Apr. 24, 2025

Clinical and Laboratory Characteristics of Chronic Viral Hepatitis C by Districts in the Samarkand Region
Yarmukhamedova Makhbuba Kudratovna 1, Rakhmonov Ravshan Nomozovich 2
1Associate Professor of the Department of Infectious Diseases and Epidemiology, Samarkand State Medical University, Samarkand, Uzbekistan
2Master's Resident of the Department of Infectious Diseases and Epidemiology, Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

According to the World Health Organization (WHO), approximately 12 million people in the European region are living with chronic hepatitis C virus (HCV). Uzbekistan has the highest HCV prevalence rate in Central Asia. In May 2022, a presidential decree was issued to implement a nationwide viral hepatitis elimination program in Uzbekistan, with the necessary funding allocated. Within the framework of this program, it is planned to screen 500,000 people annually for viral hepatitis from 2022 to 2025, ensuring access to free testing and affordable treatment. Therefore, an in-depth study of the clinical and laboratory characteristics of chronic viral hepatitis C, as well as an analysis of the existing barriers and opportunities in hepatitis screening, is crucial for the effective implementation of these initiatives.
Keywords: WHO, HCV, HBV, ELISA, PCR, Elastometry, Liver cirrhosis, Hepatocellular carcinoma
Cite this paper: Yarmukhamedova Makhbuba Kudratovna , Rakhmonov Ravshan Nomozovich , Clinical and Laboratory Characteristics of Chronic Viral Hepatitis C by Districts in the Samarkand Region, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 1181-1183. doi: 10.5923/j.ajmms.20251504.66.
Article Outline
1. Introduction
- Viral hepatitis poses a serious global public health threat. In particular, the hepatitis C virus (HCV) can lead to chronic conditions, ultimately resulting in liver cirrhosis and liver cancer, which are the most common causes of hepatitis-related deaths [1,2,3,4,9,10,11]. Today, chronic viral hepatitis C is widespread worldwide, contributing to a significant number of deaths. According to the World Health Organization (WHO), approximately 50 million people globally are infected with chronic hepatitis C, with an estimated 1 million new cases detected annually. In 2022, hepatitis C was responsible for 242,000 deaths, primarily due to liver cirrhosis and hepatocellular carcinoma [5,6,7,12,13,14,15]. A systematic review and meta-analysis of HCV prevalence in Central Asia [6] estimated that the prevalence of HCV in Uzbekistan is 9.6%. In 2016, approximately 1.3 million people in Uzbekistan were living with HCV [5]. Subsequently, WHO developed a strategic response to chronic viral hepatitis, setting specific national targets to be achieved by 2030 [8]. WHO’s European region, including Uzbekistan, has committed to developing national strategies and action plans for the elimination of viral hepatitis [8].Given the global and regional significance of studying chronic viral hepatitis C, we have conducted a clinical and laboratory analysis of this condition in the Samarkand region, specifically in the Oqdaryo and Jomboy districts. This article presents the findings of our research on chronic viral hepatitis C in these districts.The purpose of the work: The objective of this study is to conduct a clinical and laboratory analysis of the prevalence of chronic viral hepatitis in the Oqdaryo and Jomboy districts of the Samarkand region.
2. Research Methods and Materials
- A total of 105 patients diagnosed with chronic viral hepatitis, who were permanent residents of the Oqdaryo and Jomboy districts and sought outpatient care at the Hepatology Department of the Samarkand Regional Infectious Diseases Clinical Hospital between 2019 and 2023, were included in the study. The research was conducted using the following laboratory and clinical methods: Complete Blood Count (CBC) – General blood analysis. Biochemical Blood Analysis – Assessment of liver function and metabolic markers. Enzyme-Linked Immunosorbent Assay (ELISA/IFA) – Detection of hepatitis C virus-specific antigens and antibodies. Polymerase Chain Reaction (PCR) – Confirmation of the presence of the virus and viral load quantification using the METAVIR scoring system. Elastometry (FibroScan) – Evaluation of liver fibrosis severity. Survey and Interviews – Collection of epidemiological data through interviews with patients and their close relatives to determine etiological factors and analyze clinical symptoms.
3. Analysis and Results
- The study results showed that between 2019 and 2023, 105 patients who were permanent residents of the Oqdaryo and Jomboy districts and sought medical care at the hepatology department of the Samarkand Regional Infectious Diseases Clinical Hospital were diagnosed with chronic viral hepatitis C.Through patient interviews conducted among those from these districts, the following complaints and clinical symptoms were identified: Complaints: General weakness and rapid fatigue (90%), pain and a feeling of heaviness in the right hypochondrium (40%). Objective examination findings showed that patients did not present with jaundice on the skin or mucous membranes; however, scleral jaundice was observed in 19% of cases. Upon liver palpation, hepatomegaly was detected in 6% of patients. Urine output and bowel movements were normal.Laboratory and instrumental examination results varied according to gender and age categories, revealing the following findings: A complete blood count (CBC) showed that as age increased, mild anemia (Grade 1) was detected among patients. Biochemical blood analysis revealed that liver enzyme and bilirubin levels corresponded to the degree of disease activity.A review of the outpatient records and medical histories of all 105 patients showed: 40 patients with low disease activity, 57 patients with moderate disease activity, 6 patients with high disease activity.These patients were further categorized based on three different age groups (18-44, 45-59, 60-74) and gender. Age-group distribution was as follows: 18-44 years: 38 patients (36%), 45-59 years: 33 patients (31%), 60-74 years: 34 patients (33%) (Figure 1).
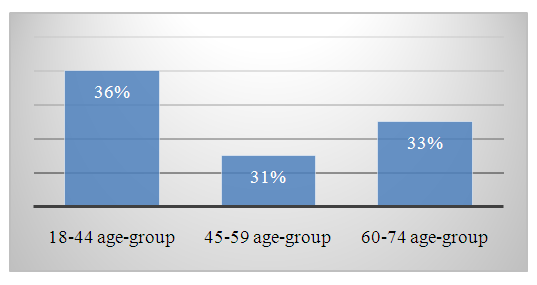 | Figure 1. Results of Patient Analysis by Age Category |
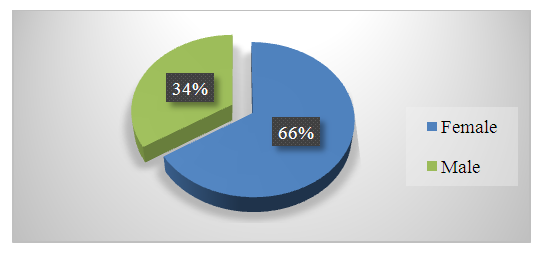 | Figure 2. Results of Patient Analysis by Gender |
|
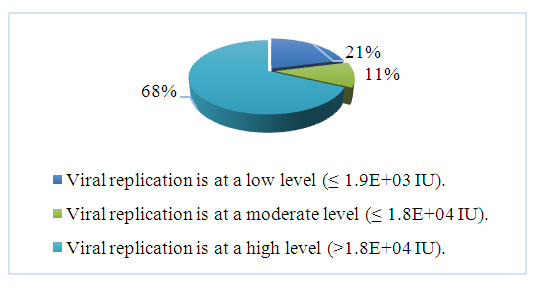 | Figure 3. The following results were obtained in patients based on PCR analysis |
4. Conclusions
- When categorizing all patients by gender, it was observed that 66% were female and 34% were male. When categorized by age group, the majority of patients were in the 18-44 age range.Fibroscan results showed that women aged 18-44 and 45-59 with F0 stage fibrosis accounted for 23% of all patients.PCR results indicated that 68% of the 105 patients had a high level of viral replication.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML