-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 1163-1168
doi:10.5923/j.ajmms.20251504.62
Received: Mar. 28, 2025; Accepted: Apr. 20, 2025; Published: Apr. 24, 2025

Immunogistochemical Aspects of Ovarian Tissue in the Dynamics of Postnatal Ontogenesis of the Generation Born on the Background of Hypotherois
Ziyoyeva Gulrukh
Assistant, Department of Anatomy and Clinical Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Ziyoyeva Gulrukh , Assistant, Department of Anatomy and Clinical Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

One of the characteristic morphological features of postnatal development in the ovaries of offspring born against the background of hypotenriosis is morphological immaturity, characterized by the presence of incompletely formed embryonic cells of the ovarian cortex and medulla. Specifically, immunohistochemical detection of these changes allows us to study the response and expression level of hormone-dependent epithelial cells through Ki-67, ER-α, and PR-α markers. It is determined that the moderate and low positive expression of ER-α and PR-α markers is manifested by the absence of a tolerance-like response in the cells of the cortex and medulla of the ovary, which lag behind in development due to inappropriate timing.
Keywords: Hypothyroidism, Immunohistochemical changes, Ovary, Postnatal ontogeny
Cite this paper: Ziyoyeva Gulrukh , Immunogistochemical Aspects of Ovarian Tissue in the Dynamics of Postnatal Ontogenesis of the Generation Born on the Background of Hypotherois, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 1163-1168. doi: 10.5923/j.ajmms.20251504.62.
Article Outline
1. Introduction
- Currently, the problem of infertility has not only medical, socio-demographic, but also economic significance. The number of infertile marriages is 10-15% of married couples and does not have a tendency to decrease. The proportion of infertility of endocrine genesis is 35-45%. Every year, an average of 4.8-10.6% of infertility cases among women of reproductive age are detected worldwide due to hypofunctional state of the endocrine system, which develops under the influence of unknown etiological factors [1,2,3]. This is explained by the fact that the thyroid gland is dysfunctional. In the USA and Europe, this problem is detected in an average of 7.2% of all reproductive women [4]. The USA spends an average of $ 4.8 billion from the state budget annually to solve this problem. In the CIS countries, this indicator averages 10.6%, and in many cases, hormonal disorders, namely chronic thyroid insufficiency, remain one of the main factors in organizing maternal and child health. In our country, an average of 980 billion soums are spent annually on the prevention of hypofunctional conditions associated with thyroid function (according to the Uzbek State Statistics Service for 2022).Nowadays, environmental pollution by electromagnetic sources is an integral part of human life. Living organisms are constantly exposed to electromagnetic radiation (EMR) of man-made origin and natural electromagnetic fields (cosmophysical factors). In recent years, mobile communication devices have been very actively introduced into people's lives, and as a result of their use, people are exposed to radiation on a daily basis. This man-made effect is not controlled and may have a cumulative nature [5,6]. There are no limits on the use of mobile communication devices. Mobile communication devices are used not only by adults, but also by children and pregnant women, exposing the still-unformed body of their unborn child to EMF, which can be very sensitive to the effects of electromagnetic radiation. Analysis of the scientific literature on the effects of EMN on the development of animals and humans gives conflicting information, but most researchers note the negative effects of cellular radiation on the reproductive system. However, other researchers found that exposure to EMN for 2 hours a day for 10 months did not affect tissue apoptosis of rat ovaries [7,8,9].That is, drawing a final conclusion based on the scientific data presented regarding the effects of EMFs from mobile phones on the reproductive system and the body as a whole is a very complex process. If the majority of researchers are on the side of the negative effects of pulsed-modulated radiation on the body, then the possible effects of EMN on the early postnatal indicators of the body's development The paucity of data on the contribution of negative impacts to the number of cases indicates the urgency of the problem and the need for selected research work at this time [10].
2. The Purpose of the Research
- The purpose of the study is to study the immunohistochemical aspects of the ovarian tissue and blood vessels of offspring born from mother rats with hypothyroidism.
3. Materials and Methods
- Ovarian tissues of 120 rats developed in the background of hypothyroidism were taken as research subjects. The obtained material was examined by statistical, morphological hemotoxylin eosin methods and morphometric methods.
4. Results and Discussion
- In the study, ovaries of 7, 14, and 21-day-old rats were obtained. Immunohistochemical studies were performed to determine the morphological and morphometric aspects of the development and maturation of the ovaries of hypothyroid rats during postnatal ontogenesis. The study examined the expression levels of the Ki-67 marker, which indicates the proliferative activity of ovarian tissue cells during development, and markers expressing estrogen (ER-α) and progesterone (PR-α) receptors to assess the morphofunctional state of ovarian cells.Ki-67 is a cell proliferation marker that is expressed to varying degrees in all active phases of the cell, G1, S, G2, and M. This marker is highly expressed from the initial phase of cell activation, G1 to M, and is most prominent in the metaphase of mitosis. In the initial phase of G1, the Ki-67 marker is localized at the centromere of satellite DNA and at the telomere of the chromosome. In the middle phases of cell activation, the Ki-67 marker is detected intranuclearly in the nucleolus, but by the G2 phase it is expressed in the nucleolus and karyoplasm. When the cell enters G0 after mitosis, the Ki-67 marker is degraded by proteosomes and undergoes complete catabolism and is no longer expressed in interphase cells. As a result, since the immunohistochemical marker indicates cell proliferation, the changes are not only indicative of tumor processes, but also of the postnatal ontogenesis of organs and tissues, which continues with cell proliferation.The Ki-67 marker can be used to calculate the cell proliferation index. A special command on the QuPath-0.5.0 platform, a modern software for calculating, allows you to count cells expressed without human factors at 200x and 400x magnification with 98.5% accuracy. This is determined by determining the percentage of all cells that are positively expressed in the nucleus. 1) 10% is considered low-level expression, 2) 10-20% is considered moderate-level expression, and 3) 20% or more is considered high-level expression. Immunohistochemical examination detects protein receptors located on the surface of cells sensitive to estrogen and progesterone, antigens using specially labeled antibodies.Since the ovarian epithelium is derived from the celiac epithelium, it develops receptors that are sensitive to specific estrogen and progesterone hormones, depending on the level of sex hormones. Detection of these receptors by antigen-antibody reaction in immunohistochemical examination, their positive staining with a specially labeled antibody, confirms the presence of these receptors and the differentiation of epithelial cells in the developing ovary. The results of this immunohistochemical method are evaluated using the following accepted terms: “strong positive reaction”, “false positive reaction”, “negative reaction” and “false negative reaction”. The percentages of low, moderate and high expression of estrogen (ER-α) and progesterone (PR-α) receptors in ovarian tissue were determined. On days 7, 14, and 21 of the first postnatal period, the proliferative index of the immunohistochemical marker Ki-67 of ovarian epithelial cells was 2.89±0.21 (in the control group, this indicator was 24.81±0.11), confirming that this indicator is low. The proliferation index of Ki-67, an immunohistochemical marker of connective tissue mesenchymal cells of the ovarian interstitial tissue, was significantly higher (41.13±0.98) than in the control group (33.15±1.03).Morphologically, the majority of histiocytic cells surrounding ovarian follicles expressed the dark brown Ki-67 marker in their nuclei. The expression of Ki-67 in the karyoplasm and nucleoli of epithelial cell nuclei also confirms that the cells are in the active G2 phase at low levels.
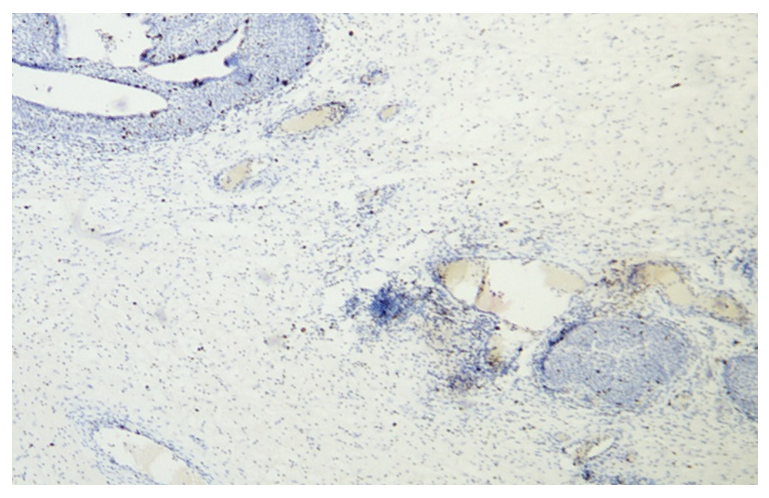
 | Figure 1. 7 days postpartum. In ovarian tissue, Ki-67 is expressed at a low level in epithelial cells and at a higher level in stromal cells. Staining: dabrochromogen. Magnification: 10X20 |
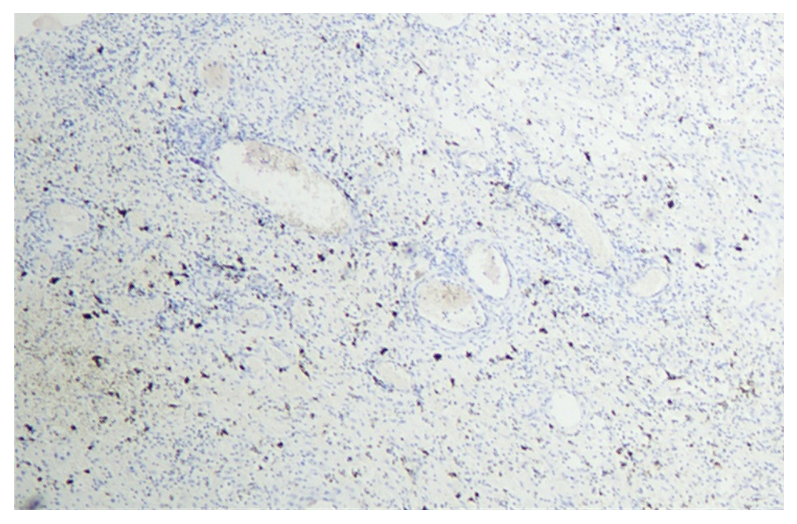
 | Figure 3. The expression level of Ki-67 marker in the epithelium of ovaries and follicles during the 14-day period. Paint: dab chromogenic. Magnification: 10x40 |
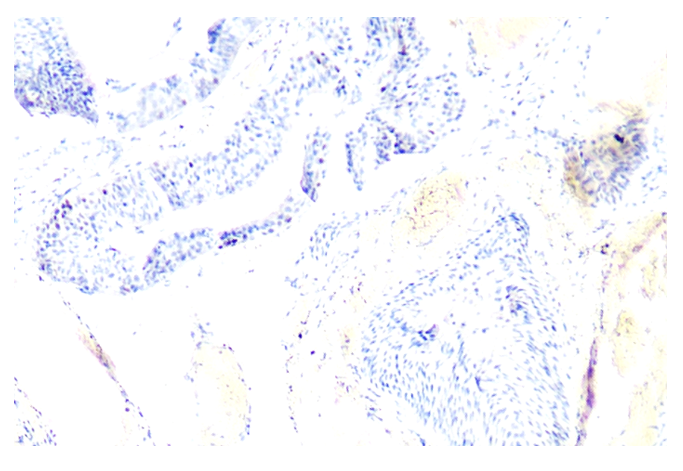
 | Figure 4. The expression level of Ki-67 marker in the epithelium of ovaries and follicles during the 14-day period. Paint: dab chromogenic. Floor: 10x40. Paint: dab chromogenic. Magnification: 10x20 |
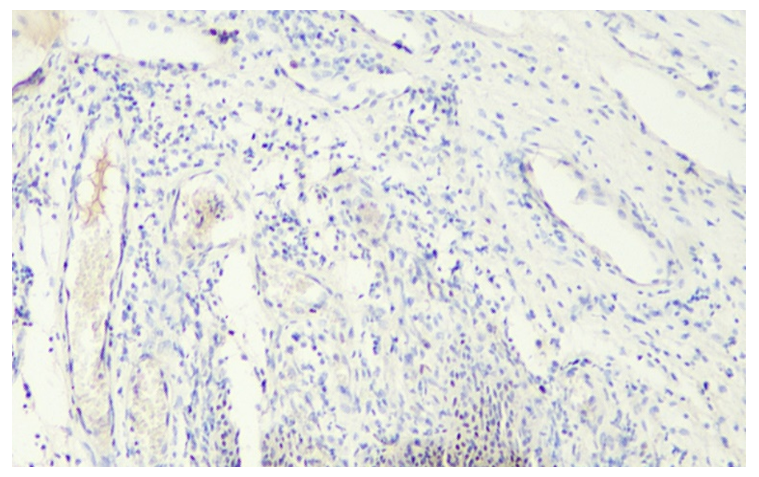
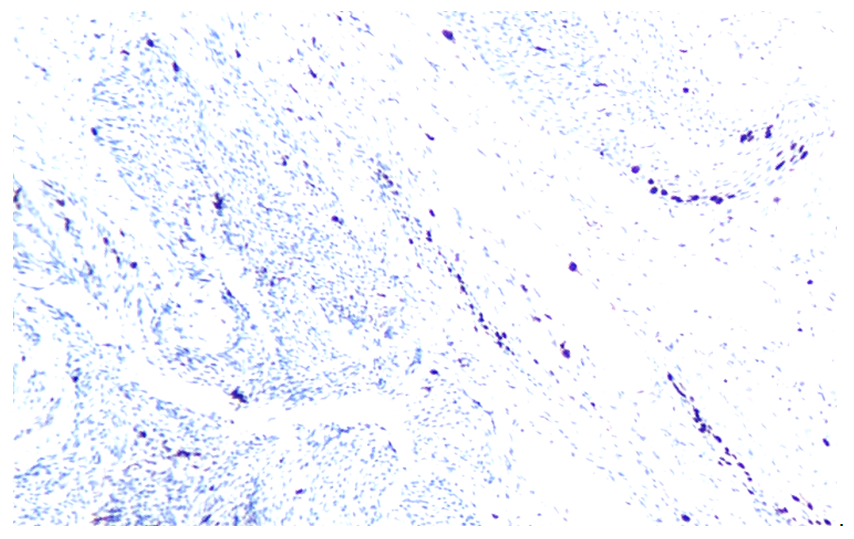 | Figure 5. Expression level of Ki-67 marker in ovarian and follicular epithelium at 21 days. Stain: dab chromogen. Magnification: 10x40 |
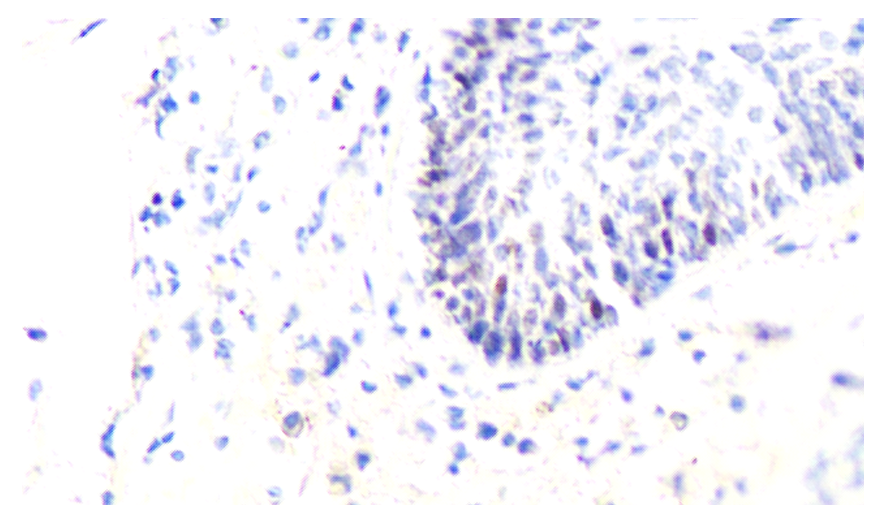 | Figure 6. Expression level of Ki-67 marker in ovarian and follicular epithelium at 21 days. Stain: Dab chromogen. Magnification: 10x40 |
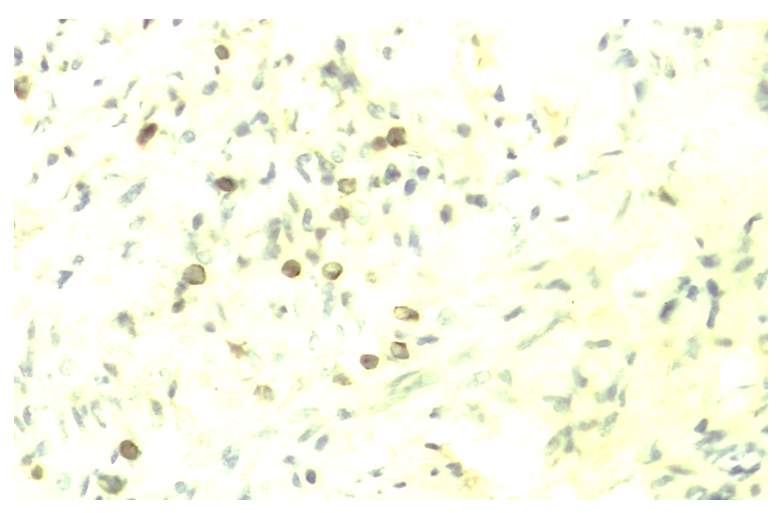 | Figure 7. The expression level of Ki-67 marker in the epithelium of ovaries and follicles during the 30-day period. Paint: dab chromogenic. Floor: 10x40. Paint: Dab chromogenic. Magnification: 10x40 |
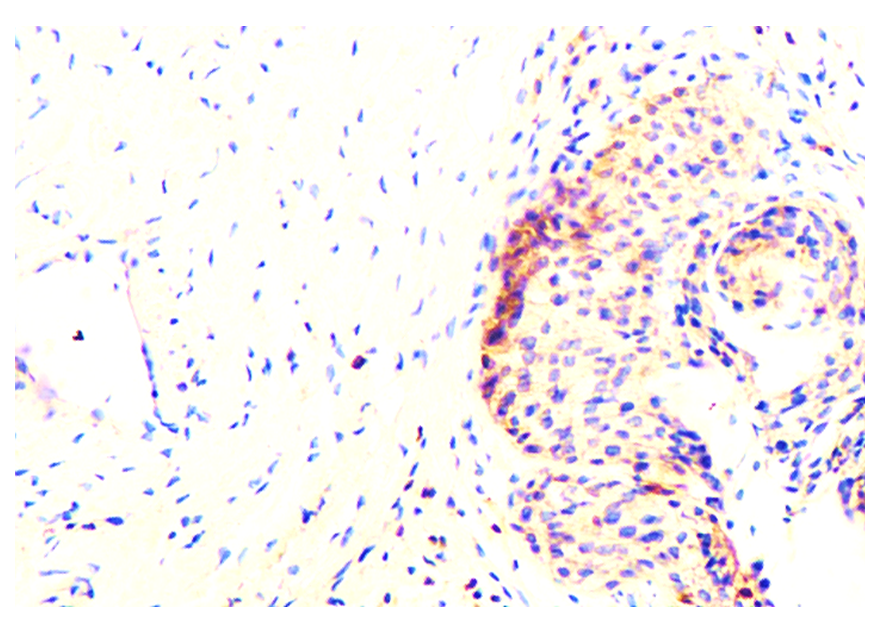 | Figure 8. The expression level of Ki-67 marker in the epithelium of ovaries and follicles during the 30-day period. Paint: dab chromogenic. Floor: 10x40. Paint: Dab chromogenic. Magnification: 10x40 |
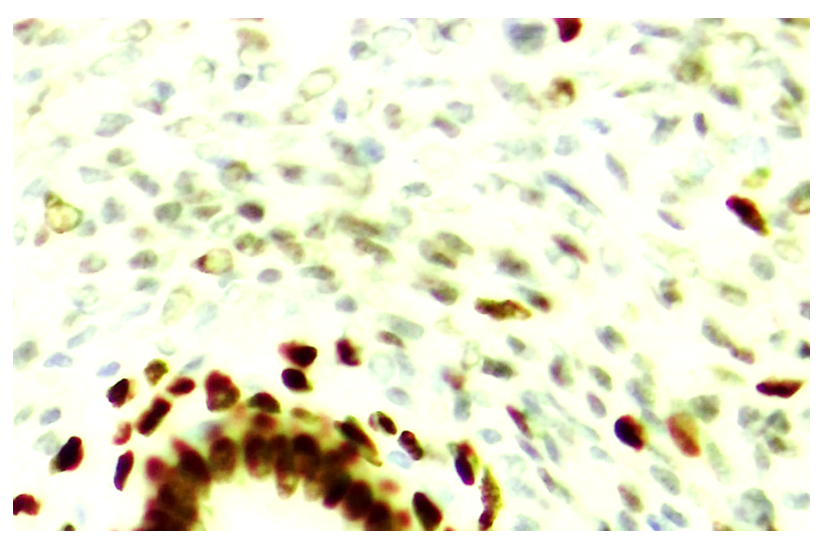 | Figure 9. Strong expression of ER-α marker in ovarian epithelium at 7 days, low expression in stroma. Paint: Dab chromogenic. Magnification: 10x40 |
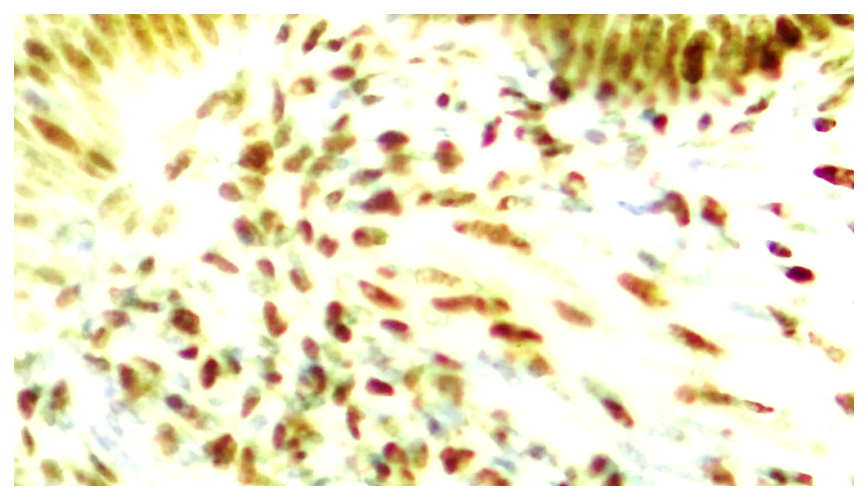 | Figure 10. Strong expression of ER-α marker in ovarian epithelium at 7 days, low expression in stroma. Paint: Dab chromogenic. Magnification: 10x40 |
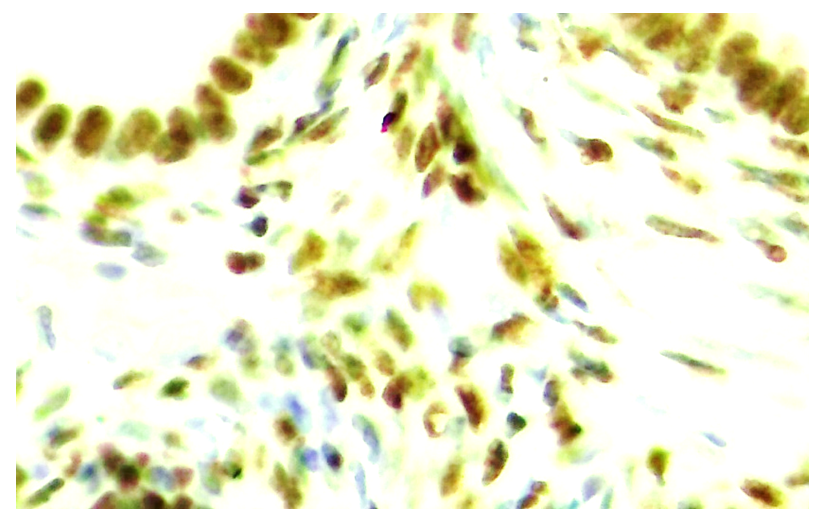 | Figure 11. 30-day cycle. Expression of progesterone receptor (PR-α) in both epithelial and stromal cells in the ovary. Paint: Dab chromogenic. Magnification: 10x40 |
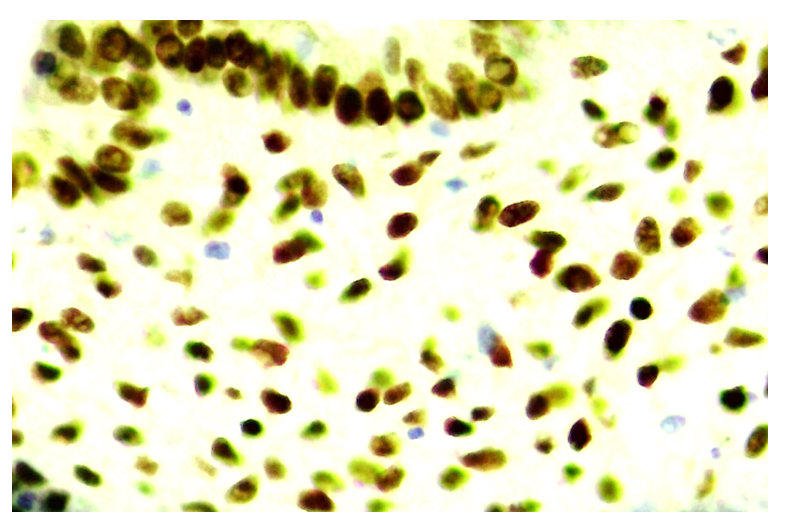 | Figure 12. 30-day cycle. Expression of progesterone receptor (PR-α) in both epithelial and stromal cells in the ovary. Paint: Dab chromogenic. Magnification: 10x40 |
5. Conclusions
- According to the results of immunohistochemical examination of ovarian tissue, the expression of estrogen (ER-α) and progesterone (PR-α) receptors in both follicular epithelial cells and stromal structures is variable, this indicates that tissue structures have different levels of sensitivity to hormones and the emergence of special protein-containing receptors during postnatal ontogenesis. The higher expression level of the ER-α receptor in follicular epithelial cells compared to the PR-α receptor in stromal structures indicates that the role of the estrogen hormone in the development mechanism of the follicular epithelium in ovarian tissue is of great importanceA deeper study of the composition of these receptors at the level of molecular biology will help to determine the macromolecular reactions that underlie the pathogenesis of endometriosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML