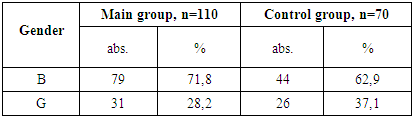-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 1159-1162
doi:10.5923/j.ajmms.20251504.61
Received: Mar. 24, 2025; Accepted: Apr. 16, 2025; Published: Apr. 24, 2025

Viral Diarrhea in HIV-Positive Children: Clinical and Immunological Features
Otajanov Shamsiddin Zarifbayevich
Assistant, Department of Infectious Diseases, Epidemiology and Phthisiology, Urganch Branch of Tashkent Medical Academy, Uzbekistan
Correspondence to: Otajanov Shamsiddin Zarifbayevich , Assistant, Department of Infectious Diseases, Epidemiology and Phthisiology, Urganch Branch of Tashkent Medical Academy, Uzbekistan.
| Email: |  |
Copyright © 2025 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The virological and immunological characteristics and changes in HIV-positive children with viral diarrhea were studied and analyzed in this article. Viral diarrhea is a significant cause of morbidity and mortality in HIV-positive children, exacerbated by their compromised immune systems. This review explores the clinical and immunological features of viral diarrhea in pediatric HIV patients, focusing on common pathogens such as rotavirus, norovirus, and adenovirus. Clinical manifestations often include prolonged and severe diarrhea, dehydration, and malnutrition, which contribute to worse outcomes compared to HIV-negative children. Immunologically, the depletion of CD4+ T cells and impaired mucosal immunity in HIV-infected children increase susceptibility to persistent and recurrent viral infections. Understanding these clinical and immunological dynamics is crucial for improving diagnostic, therapeutic, and preventive strategies in this vulnerable population. Further research is needed to optimize management and reduce the burden of viral diarrhea in HIV-positive children.
Keywords: HIV infection, Diarrhea, Rotavirus, Adenovirus, Norovirus
Cite this paper: Otajanov Shamsiddin Zarifbayevich , Viral Diarrhea in HIV-Positive Children: Clinical and Immunological Features, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 1159-1162. doi: 10.5923/j.ajmms.20251504.61.
Article Outline
1. Introduction
- Today, the global spread of HIV remains one of the greatest threats to human security. This issue was officially acknowledged by the United Nations General Assembly in its founding document, the “Declaration of Commitment on HIV/AIDS.” According to the World Health Organization (WHO), by 2021, approximately 38.4 million people were living with HIV worldwide, including 1.7 million children. Newborns can contract HIV from their mothers in three ways: during pregnancy, at the time of birth, and through breastfeeding [1,5,7].Acute intestinal infections also remain a pressing public health concern due to their widespread nature, severe clinical course, potential complications, and their tendency to cause long-term gastrointestinal disorders. While bacterial infections are more familiar to many healthcare providers, viruses are responsible for 30–40% of acute diarrhea episodes in young children, with rotavirus being the leading cause in 60–80% of cases. In immunocompromised individuals—such as organ transplant recipients, cancer patients receiving chemotherapy, and individuals with HIV/AIDS—viral infections of the gastrointestinal tract can lead to serious complications and even death, despite adequate medical care. Recently, reports of viral diarrhea caused by non-rotavirus agents have increased, prompting the need to better understand the main viruses responsible for epidemic outbreaks of acute gastroenteritis.Viral diarrhea often begins suddenly and can rapidly lead to dehydration. If properly treated with timely rehydration, outcomes are usually positive. However, these viruses show strong resistance to environmental conditions and remain highly contagious even when containment measures are applied. Patients may continue shedding the virus even after recovering clinically, and there is ongoing debate about potential aerosol transmission. Dehydration occurs rapidly due to the breakdown of disaccharides and lipids without changes in adenyl cyclase or sAMF levels, leading to damage of intestinal cells and resulting in secondary lactose intolerance [5,8,11].In HIV-infected individuals, many pathogens can cause enteritis or enterocolitis, leading to acute, chronic, or recurrent diarrhea. In children, common culprits include Salmonella spp., Shigella spp., Campylobacter jejuni, Giardia lamblia, Cryptosporidium parvum, cytomegalovirus, adenovirus, rotavirus, and herpes simplex virus. Studies show that viruses are responsible for around 80% of intestinal infections in children. Rotavirus accounts for up to 50% of cases, and norovirus for around 30% in developed countries. The range of identified viral agents continues to expand, now including astrovirus, adenovirus, and torovirus [4,7,14].Monitoring liver enzymes, pancreatic enzyme activity, and bilirubin levels is essential to ensure the safety of antiretroviral therapy. If signs of hepatitis or pancreatitis emerge during treatment, it’s necessary to switch medications. Gastrointestinal damage in HIV-infected children plays a key role in the disease’s clinical picture and influences both its progression and outcome. These children are prone to diarrhea due to intestinal changes triggered by various fecal-oral infections. Dysfunction of the gastrointestinal system has a major impact on their nutrition and immune function, which can impair growth, physical development, and even central nervous system maturation. HIV affects all parts of the digestive tract [6,7].
2. Purpose of the Research
- To study of clinical and laboratory features of viral diarrhea in HIV-infected children. The purpose of this research is to investigate the clinical and immunological characteristics of viral diarrhea in HIV-positive children, with the aim of improving understanding, diagnosis, and management of this condition.
3. Material and Methods
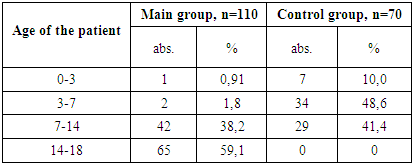
- In the course of the study, children under the age of 18 were divided into two groups. The main group included 110 HIV-positive children who were diagnosed with viral diarrhea, while the control group consisted of 70 children with viral diarrhea but without HIV infection.The diagnosis of HIV in children was carried out in accordance with official guidelines: the "National Clinical Protocol for the Organization and Delivery of Medical Care to Individuals with Confirmed HIV Status" (Order No. 206 dated August 19, 2021, issued by the Ministry of Health of the Republic of Uzbekistan), and Order No. 122 dated March 25, 2015, "On Measures to Improve the Control of Typhoid Fever, Paratyphoid Fever, Salmonellosis, and Acute Intestinal Infections."
4. Results and Discussion
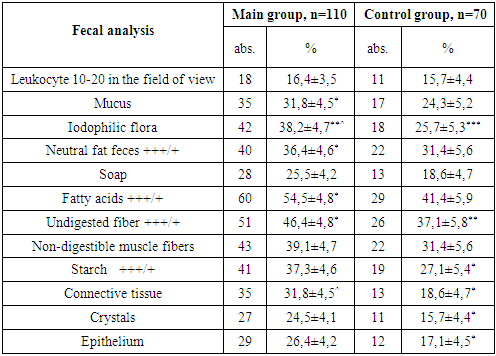
- To evaluate the severity of acute infectious diarrhea in HIV-infected children, several factors were taken into account. These included the degree of dehydration (as defined by WHO criteria), the frequency and duration of diarrhea episodes, as well as the physical characteristics of the stool—such as form, consistency, odor, color, volume, and the presence of pathological inclusions. Diagnoses were established through a combination of patient-reported symptoms, clinical examinations, anthropometric measurements, and laboratory analyses including serological, bacteriological, immunological, virological, and instrumental diagnostic methods.
|
|
|
|
|
|
|
5. Conclusions
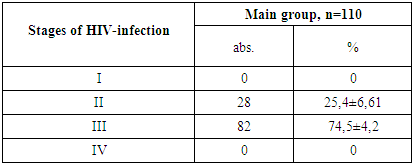
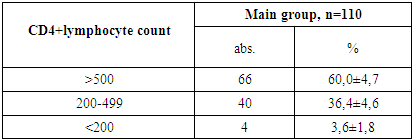
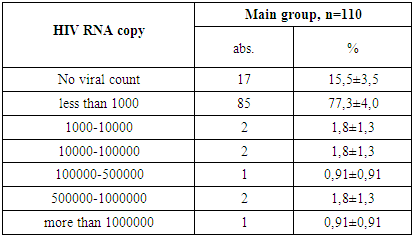
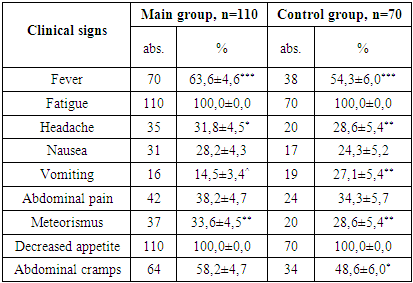
- 1. Gender Distribution: In both the main and control groups, the disease was more prevalent among boys, accounting for 71.8% of cases in the main group and 62.9% in the control group.2. Clinical Stage of HIV: Among patients in the main group, only clinical stages II and III of HIV infection were identified, occurring in 25.4% and 74.5% of cases, respectively.3. Immunological Characteristics: Immunological assessment revealed that severe immunodeficiency was rare in the main group, found in only 3.6% of patients. Additionally, the majority of these children (77.3%) had a low viral load, with HIV RNA levels below 1,000 copies/mL.4. Clinical Symptoms: Weakness and loss of appetite were universal symptoms, observed in 100% of patients across both groups. Fever was the most common sign of intoxication, reported more frequently in the main group (63.6%) than the control group (54.3%). Headaches were noted in roughly one-third of patients in both groups.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML