-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 1063-1066
doi:10.5923/j.ajmms.20251504.43
Received: Mar. 23, 2025; Accepted: Apr. 9, 2025; Published: Apr. 12, 2025

Study of Comorbid States in Patients with Chronic Heart Failure: Impact on Prognosis and Treatment
Erkinova Nigora Erkinovna
PhD, Associate Professor, Department of Phthisiology and Pulmonology, Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Erkinova Nigora Erkinovna, PhD, Associate Professor, Department of Phthisiology and Pulmonology, Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chronic heart failure (CHF) is an important and pressing medical problem, being one of the leading causes of death among the world's population. According to the World Health Organization, about 17 million people die annually from the consequences of cardiovascular diseases (CVD). CHF is a consequence of various cardiac diseases, such as coronary heart disease (CHD), hypertension and cardiosclerosis. In recent years, special attention has been paid to comorbid conditions that arise against the background of the underlying disease and have a significant impact on the course of the disease, its prognosis and the quality of life of patients. Among the many comorbid conditions, special attention should be paid to microalbuminuria, which is an early marker of renal damage and can predict the deterioration of CHF. Recent studies show that the presence of albuminuria and other renal fibrosis markers significantly worsen the prognosis and increase mortality among patients with CHF. This fact emphasizes the need for a comprehensive approach to the treatment of CHF, taking into account the impact of comorbid diseases, especially kidney diseases. Indeed, comorbid conditions are most often observed in CHF, accounting for an average of 92%. A high level of comorbidity leads to an increase in mortality from chronic diseases, a decrease in the quality of life and a violation of social adaptation. Therefore, timely diagnosis of comorbid diseases, their comprehensive treatment and prevention are urgent problems of medicine in CHF. At present, the relationship between microalbuminuria or single proteinuria and the course of cardiovascular diseases, as well as increased mortality from these diseases, has not been sufficiently studied. However, there is evidence that the presence of albuminuria in patients with CHF significantly worsens the course of the disease and is one of the leading factors leading to death. This is especially evident in the presence of comorbid conditions. Some scientific observations confirm that microalbuminuria (proteinuria) is an early and sensitive marker of renal damage in patients with CHF, which can be tracked in relation to the level of creatinine in the blood.
Keywords: Chronic heart failure, Diagnostics, Comorbid condition
Cite this paper: Erkinova Nigora Erkinovna, Study of Comorbid States in Patients with Chronic Heart Failure: Impact on Prognosis and Treatment, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 1063-1066. doi: 10.5923/j.ajmms.20251504.43.
Article Outline
1. Introduction
- Atrial fibrillation (AF) and chronic heart failure (HF) are major contributors to cardiovascular morbidity and mortality, with growing evidence highlighting the interplay between cardiac dysfunction, renal impairment, and systemic inflammation. Mineralocorticoid receptor antagonists (MRAs), such as eplerenone, have demonstrated beneficial effects in HF with reduced ejection fraction (HFrEF), including reductions in atrial fibrillation and improvements in myocardial fibrosis, functional capacity, and quality of life [1,2]. However, the role of MRAs in HF with preserved ejection fraction (HFpEF) remains less clear, particularly in patients with concurrent chronic kidney disease (CKD), who face an elevated risk of adverse cardiovascular outcomes [3,4]. The cardiorenal interaction is a critical determinant of prognosis, as CKD exacerbates cardiac structural remodeling and contributes to the progression of HF [5,6]. Additionally, inflammatory pathways, including interleukin-6 (IL-6) signaling, have been implicated in the pathophysiology of HF, particularly in patients undergoing cardiac resynchronization therapy [7,8]. Subclinical renal damage, such as microalbuminuria, further underscores the systemic nature of cardiovascular disease and its association with endothelial dysfunction [8]. Given the complex interplay between cardiac, renal, and inflammatory mechanisms, a deeper understanding of the effects of MRAs on these pathways is essential, particularly in high-risk populations with overlapping HF and CKD. This study seeks to explore the potential benefits of eplerenone in modulating myocardial fibrosis, functional status, and inflammatory markers in patients with HF and renal impairment, while addressing gaps in current evidence regarding its role in HFpEF and cardiorenal syndromes [9].
2. Purpose of the Research
- The aim of this study was to investigate the relationship between albuminuria, comorbid diseases and renal fibrosis indices in patients with CHF. We investigated the impact of these factors on the clinical status, physical endurance and quality of life of patients.
3. Materials and Methods
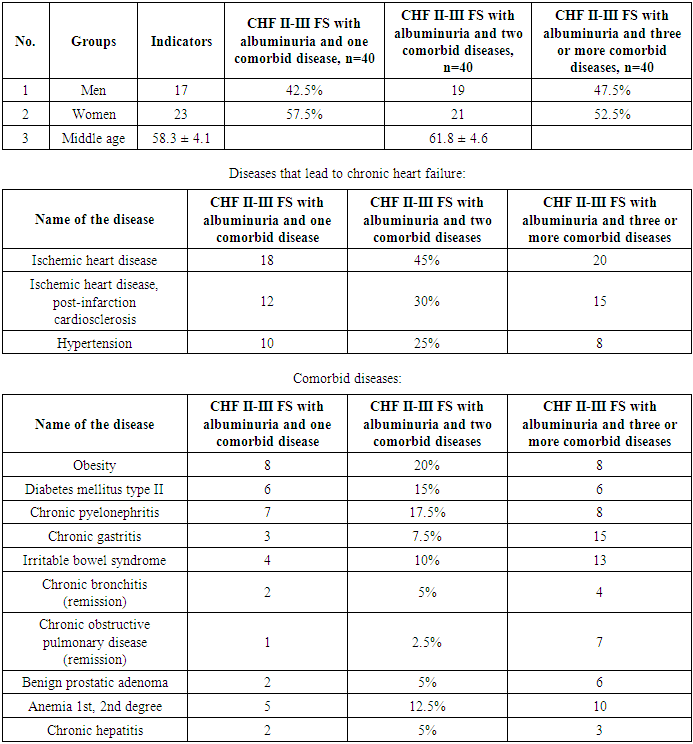
- The study was conducted at the cardiology department, where 320 patients diagnosed with chronic heart failure stage II-III of the functional class (FS) were observed. Of these, 148 (46.25%) were men and 173 (53.57%) were women. The average age of patients was 61.4 ± 5.6 years. A total of 120 patients with CHF were divided into three groups. The first group consisted of 40 patients with CHF stage II-III FS, who were diagnosed with albuminuria and one comorbid disease. The average age of this group was 58.3±4.2 years, of which 17 were men and 23 were women. The second group also included 40 patients with CHF stage II-III FS and albuminuria, but with two comorbid diseases. The average age of these patients was 61.8±4.7 years, of which 19 were men and 21 were women. The third group consisted of 40 patients with CHF stage II-III FS and albuminuria, who had three or more comorbid diseases. The average age of this group was 65.9±5.3 years, of which 21 were men and 19 were women. In all cases, the cause of CHF development was ischemic heart disease, post-infarction cardiosclerosis, and hypertension. In some cases, it was found that ischemic heart disease and hypertension simultaneously caused CHF in one patient, which was recorded in the anamnesis and objective examination. Also, 120 patients with CHF had obesity and diabetes mellitus, and these diseases were evenly distributed across all groups.All patients received standard treatment for CHF, including β-blockers, angiotensin II receptor antagonists such as azilsartan, and latest-generation antifibrotic agents such as eplerenone (25-50 mg). In some cases, cardiac glycosides, diuretics, and antiarrhythmics were prescribed. All patients had their blood potassium levels and glomerular filtration rate (>60 ml/min per 1.73 m² of body surface) monitored. In cases of hyperkalemia, eplerenone was discontinued.
4. Results and Discussion
- Information about the patients included in the study, as well as the identified comorbid diseases, is presented in Table 1.
|
5. Conclusions
- The presence of comorbid diseases in chronic heart failure negatively affects the clinical condition of patients, increasing the level of fibrosis markers and worsening physical endurance and quality of life. An increase in the number of comorbid diseases significantly worsens the prognosis and increases mortality among patients with CHF. Early detection and timely treatment of comorbid diseases, as well as the use of markers of renal damage, such as albuminuria, cystatin C and TGF-β1, is an important part of a comprehensive approach to the treatment of CHF.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML