-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 1023-1030
doi:10.5923/j.ajmms.20251504.35
Received: Mar. 9, 2025; Accepted: Mar. 29, 2025; Published: Apr. 10, 2025

Retrospective Study, Diagnosis, and Clinical Characteristics of Atopic Dermatitis in Children of the Samarkand Region
Khamidova Farida Muinovna, Tolibov Mansur Mahmudovich, Ismoilov Jasur Mardonovich
Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

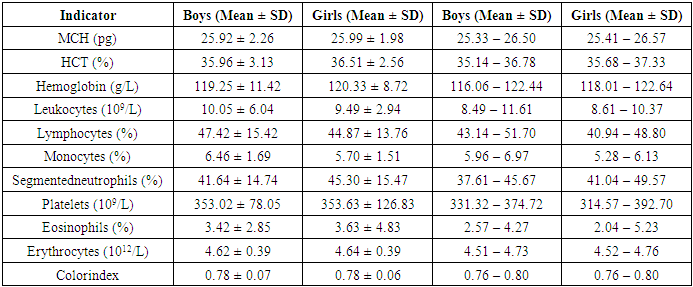
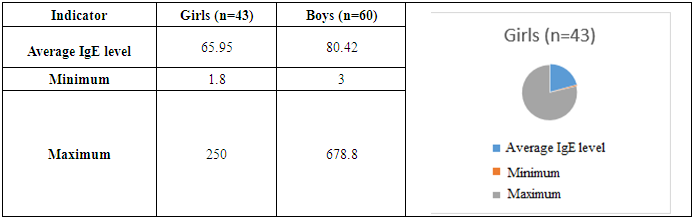
The article presents a comprehensive analysis of a retrospective, diagnostic study of atopic dermatitis in children of the Samarkand region for 2024. The inclusion and exclusion criteria of patients are thoroughly examined, confirming the validity of the selected study groups. Particular attention is paid to the comparative laboratory analysis of young children with atopic dermatitis. Atopic dermatitis is most common in the Taylak, Urgut, and Samarkand districts. Boys are more frequently affected, especially under the age of one. The incidence is seasonal, peaking in the spring and summer months. Blood test data in children with atopic dermatitis show similar results in both genders, but girls have higher levels of platelets and segmented neutrophils, while boys show higher levels of leukocytes and lymphocytes. The average IgE level in girls is 65.95, while in boys, it is 80.42, indicating a slightly higher level in boys. Boys also have higher liver enzyme and bilirubin levels, possibly due to a more active metabolism. Girls show elevated creatinine and Ritis ratio, which may reflect differences in metabolism. The remaining indicators do not show significant differences.
Keywords: Atopic dermatitis, Chronic obstructive bronchitis, Morphometry
Cite this paper: Khamidova Farida Muinovna, Tolibov Mansur Mahmudovich, Ismoilov Jasur Mardonovich, Retrospective Study, Diagnosis, and Clinical Characteristics of Atopic Dermatitis in Children of the Samarkand Region, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 1023-1030. doi: 10.5923/j.ajmms.20251504.35.
1. Introduction
- The issue of atopic dermatitis remains one of the most pressing in dermatology due to its widespread prevalence, increasing incidence, severe course, and the insufficient effectiveness of existing treatment methods [2,17,34,37]. The increase in morbidity over the past decade, the chronic nature with frequent relapses, and the insufficient effectiveness of current treatment and prevention methods place atopic dermatitis among the most significant problems in modern medicine [12,14,15,21,36,38]. Atopic dermatitis typically develops in early childhood in individuals with a hereditary predisposition to atopic diseases and has age-related features in the localization and morphology of inflammation foci.Atopic dermatitis (AD) is one of the most common inflammatory dermatoses and allergic diseases. The incidence among the population of different countries is usually not less than 5-10%, in industrialized countries around 20%, and in children, AD has long been the leading pathology [1,3,12,26,40].Currently, atopic dermatitis (AD) is understood as a chronic allergic disease characterized by exudative and lichenified rashes, elevated serum IgE levels, and hypersensitivity to specific irritants. However, as shown in several studies by domestic and foreign researchers, only some patients exhibit increased levels of total and allergen-specific IgE, which allows only these patients to be classified in the IgE-mediated type of the disease. In some patients, IgE-mediated hypersensitivity in the pathogenesis cannot be identified, but delayed-type hypersensitivity is present [4,7,11,16,17]. Besides these two groups, there are patients with a mixed type of allergic reactions in the pathogenesis. Finally, in some patients, the pathogenesis of AD is due to other, non-immune mechanisms [5,7,8,10,29].The concept of "atopy" was introduced into medical practice in 1923 by Coca and Cooke. The term initially encompassed the clinical manifestations of such externally dissimilar diseases as asthma, urticaria, hay fever, food allergies, and eczema, that is, everything that is now considered as variants of type I (immediate) hypersensitivity. In addition to immune mechanisms, all forms of atopy share the fact that they occur significantly more often in people whose parents have similar ailments. This pattern is not strict, however, some authors, who have a weakness for simple explanations of their failures, willingly interpret the term "atopy" precisely as a genetic predisposition to corresponding allergic reactions, which is equivalent to recognizing the incurability of all forms of atopy, including, of course, atopic dermatitis. Such a substitution of concepts, as will be shown below, is absolutely unfounded and brings nothing but a sense of hopelessness. The term "atopic dermatitis" is now widely accepted to refer to this disease [6,8,13,14,18,19,20,32,35].The prevalence of AD among developed countries is 10-20%. Manifestation of AD symptoms in children is observed at the age of 6 months in 60% of cases, up to 1 year in 75%, and up to 7 years in 80-90%. Over the past decades, there has been a significant increase in the incidence of AD, its course has become more complicated, and its outcome has become more severe. AD is often associated with other allergic diseases — with bronchial asthma in 34%, allergic rhinitis in 25%, and pollinosis in 8% [9,21,22,24,26,39,40].Atopic dermatitis is the main problem of pediatric dermatology, as the incidence of atopic dermatitis is much higher than other common dermatitis. The terms "atopic eczema" and "neurodermatitis" are used as synonyms for this disease. In everyday life, people simply use the term "eczema." We consider the terms "eczema" and "dermatitis" equivalent and will use exclusively the term "dermatitis" to avoid confusion [19,20,21,23,25,27,28,31].Recently, studies have begun to explore the deterioration in the quality of life of both the patient and their family — in this disease, it is quite significant. Most patients are children. The incidence of atopic dermatitis is 8-16%, making it the most common skin disease of infancy and childhood [33,34,36,38,40,41,42]. Approximately 60% of patients show the first signs of the disease in the first year of life. Males and females are affected with equal frequency.Research Aim. To conduct a retrospective, clinical, and diagnostic study of children with atopic dermatitis in the Samarkand region.
2. Materials and Methods of Research
- The study used retrospective, clinical, and morphological methods for treating patients with atopic dermatitis. The material consisted of outpatient records, clinical studies, and laboratory studies of children with atopic dermatitis.
3. Research Results
- Results of the retrospective study. Patients with atopic dermatitis were examined through retrospective observation of children. In 2024, at the Samarkand branch of the Specialized Scientific and Practical Medical Center of Dermatovenereology and Cosmetology, 42,917 thousand people consulted for dermatovenereological diseases. Of these, a clinical diagnosis of atopic dermatitis was made in 1,330 patients of different ages, which accounted for 3.1% of the total number of patients.When we conducted an analysis by district within the Samarkand region, the following was revealed:
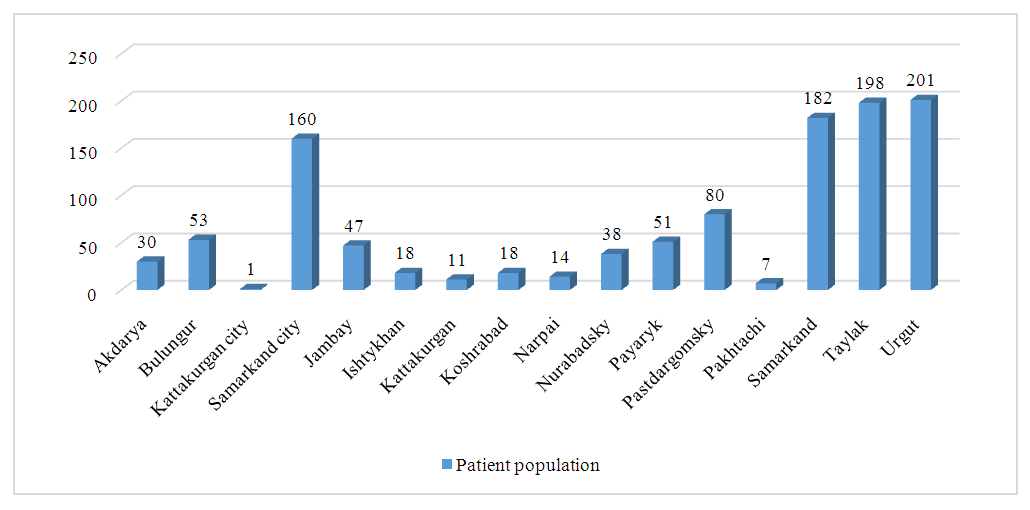 | Figure 1. Analysis of the incidence of atopic dermatitis by districts of the Samarkand region |
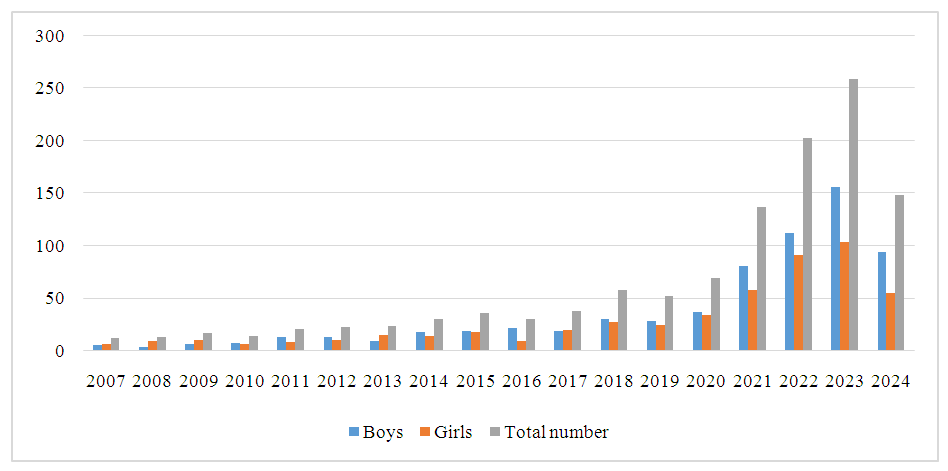 | Figure 2. Analysis of children with atopic dermatitis by gender |
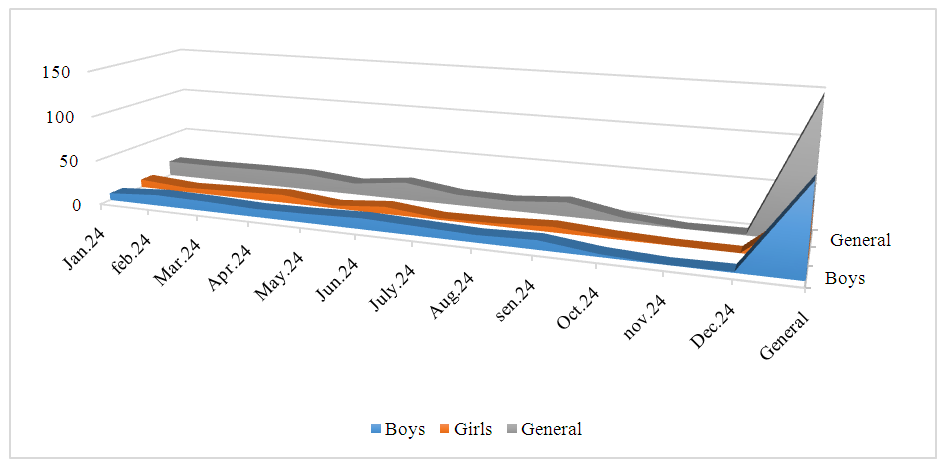 | Figure 3. Atopic dermatitis in children born in 2024 |
|
|
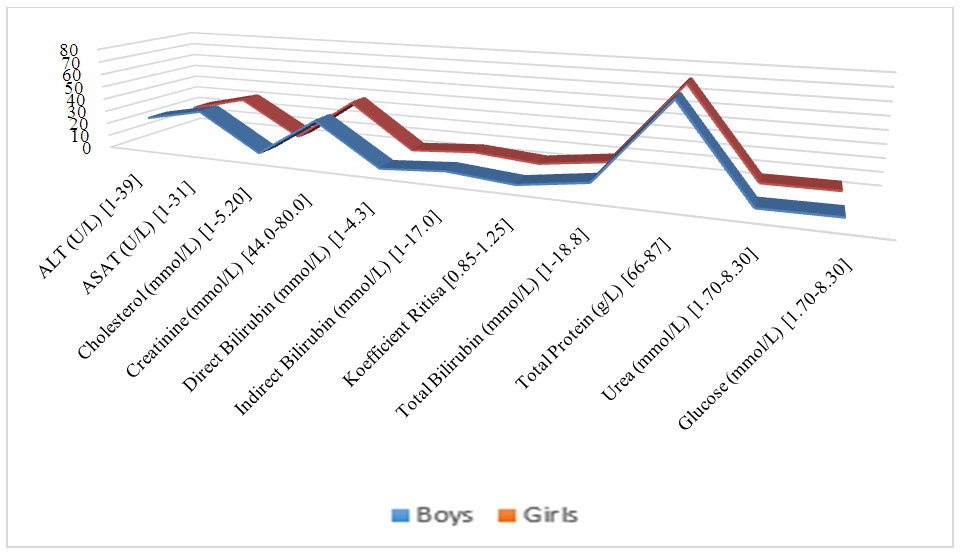 | Figure 4. Biochemical analysis indicators of children with atopic dermatitis |
4. Conclusions
- Atopic dermatitis is most common in the Taylak, Urgut, and Samarkand districts. Boys are more frequently affected, especially under the age of one. The incidence has a seasonal nature, peaking in the spring-summer period.Blood test data in children with atopic dermatitis show similar indicators in both sexes, although girls have higher platelet and segmented neutrophil levels, while boys show higher levels of leukocytes and lymphocytes. The average IgE level in girls is 65.95, while in boys, it is 80.42, indicating a slightly higher level in boys. Boys also have higher liver enzyme and bilirubin levels, likely due to more active metabolism. Girls show elevated creatinine and Ritis ratio, which may reflect differences in metabolism. The remaining indicators do not show significant differences.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML