A. A. Mamarizaev1, U. M. Rustamov1, Q. T. Boboev2, H. T. Musashayhov1, U. H. Musashayhov1
1Andijan State Medical Institute, Andijan, Uzbekistan
2Republican Specialized Scientific and Practical Medical Center of Hematology, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The Val89Leu polymorphism in the SRD5A2 gene, which encodes 5α-reductase type II, plays a critical role in androgen metabolism by converting testosterone into dihydrotestosterone (DHT). This polymorphism involves a substitution of valine with leucine at position 89, resulting in reduced enzymatic activity and lower levels of DHT. Studies suggest that this variation influences the development and progression of benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Men carrying the Leu89 allele typically exhibit a lower prostate volume, reduced risk of BPH progression, and a decreased likelihood of developing aggressive forms of PCa. However, the polymorphism may affect the efficacy of 5α-reductase inhibitors, such as finasteride, commonly used in the treatment of these conditions. Furthermore, the impact of Val89Leu varies across different populations, highlighting the need for genetic profiling in assessing individual risks and tailoring therapies. This polymorphism represents a promising biomarker for advancing personalized approaches in the prevention, diagnosis, and treatment of androgen-related prostatic diseases.
Keywords:
SRD5A2 gene, Val89Leu polymorphism, 5α-reductase type II, Dihydrotestosterone (DHT), Benign prostatic hyperplasia (BPH), Prostate cancer (PCa)
Cite this paper: A. A. Mamarizaev, U. M. Rustamov, Q. T. Boboev, H. T. Musashayhov, U. H. Musashayhov, Significance of the Val89Leu Polymorphism in the SRD5A2 Gene in the Development of Benign Prostatic Hyperplasia and Prostate Cancer, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 997-1001. doi: 10.5923/j.ajmms.20251504.31.
1. Introduction
The SRD5A2 gene, located at the 2p23 locus, encodes the protein steroid 5-alpha reductase type II (5aR2), a key enzyme in testosterone metabolism [5]. The SRD5A2 gene, located at the 2p23 locus, encodes the protein steroid 5-alpha reductase type II (5aR2), a key enzyme in testosterone metabolism [7]. This enzyme belongs to the NADPH-dependent enzyme and converts testosterone into dihydrotestosterone, which is 10 times more active than its predecessor. SRD5A2 is significantly present in androgen-sensitive tissues [3,2]. Based on in vitro studies, it has been shown that the polymorphic variant reduces the activity of the enzyme by 40% [6]. In European men, the homozygous variant reduces testosterone levels by 12% and free testosterone by 16% [4,8]. Deficiency of steroid 5-alpha-reductase type 2 (5αRD2) is a rare autosomal recessive disorder caused by mutations in the SRD5A2 gene [9,10]. A defect in the 5-alpha reductase enzyme, which ensures conversion of testosterone into dihydrotestosterone, leads to disorders of sex development [1,6]. In this study, we studied the distribution of allele and genotype frequencies of the polymorphic marker Val89Leu in the SRD5A2 gene in the main group of patients and in the comparison group. At comparative analysis of major allele A of genetic marker Val89Leu in SRD5A2 gene, we found no significant differences between patients and control group (63.7% and 65.2%, respectively; OR=0.9; 95%CI:0.63-1.39; χ2=0.1; p=0.8). Further analysis of the minor allele G of this polymorphism also showed differences in frequency of occurrence between patients and controls (36.3% and 34.8%, respectively; OR=1.1; 95%CI:0.72-1.59; χ2=0.1; p=0.8), but these differences did not meet the level of significance required to be considered statistically significant [9]. When analyzing the obtained results, it was revealed that in the main sample of patients there were no significant differences in the frequency of occurrence of genotypes A/A and A/G of Val89Leu polymorphism in SRD5A2 gene in comparison with practically healthy individuals. Thus wild A/A genotype in the main group of patients was found with OR=0.9; 95%CI:0.55-1.63; χ2=0.0; p=0.9 in 44.3%, and in the reference group this genotype was found in 45.7% of cases. In turn, the heterozygous A/G genotype was found less frequently in the reference group compared to the virtually healthy individuals (38.7% vs. 39.0%, respectively, OR=1.0; 95%CI:0.57-1.71; χ2=0.0; p=0.98), but the differences did not reach true significance (see Table 1).Table 1. Carriage of alleles and genotypes of Val89Leu polymorphism in SRD5A2 gene in the main group of patients and controls
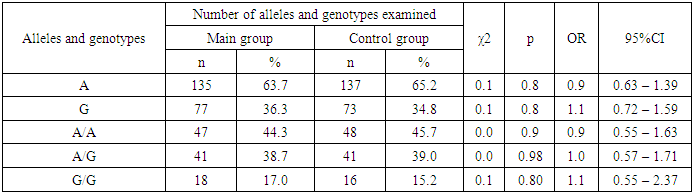 |
| |
|
Investigation of the mutation in the SRD5A2 gene (Val89Leu) by mutant genotypes showed that the G/G mutant genotype was detected in 17.0% of patients, which is insignificantly higher than in the comparison group, where this mutation was found in 15.2% of cases (with χ2<3.84, p>0.05).The study of the allele frequency of the Val89Leu polymorphism in the SRD5A2 gene in patients with BPH and in conventionally healthy individuals revealed insignificant differences (see Table 2).Table 2. Carriage of alleles and genotypes of Val89Leu polymorphism in SRD5A2 gene in the main group of patients with BPH and controls
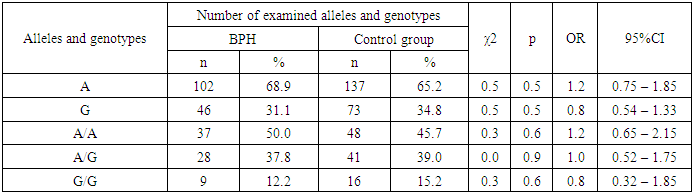 |
| |
|
The study of the distribution of alleles A (68.9% vs. 65.2%) and G (31.1% vs. 34.8%) above the indicated polymorphism showed that the presence of these alleles of major and minor type did not have a predisposing significance to the development of BPH in relation to the control (χ2=0.5; p=0.5; OR=1.2; 95%CI:0.75-1.85 and χ2=0.5; p=0.5; OR=0.8; 95%CI:0.54-1.33). In the group of studied patients with BPH, it was found that the genotypes of Val89Leu polymorphism in the SRD5A2 gene had a distribution similar to the allelic distribution. Comparing with the group of patients with BPH, the frequency of G/G genotype was higher in the group of healthy individuals (12.2% vs. 15.2% at χ2=0.3; p=0.6; OR=0.8; 95%CI:0.32-1.85). At the same time, the frequency of A/A genotype was lower in the group of conventionally healthy individuals (45.7%) compared to the group of patients with BPH (50.0%), but the difference did not reach statistical significance (χ2=0.3; p=0.6; OR=1.2; 95%CI:0.65-2.15). Although the frequency of the heterozygous A/G genotype was higher in the reference group relative to the patients with DGPH, the results also did not reach the statistical significance necessary to be considered reliable (39.0% vs. 37.8%, χ2=0.0; p=0.9; OR=1.0; 95%CI:0.52-1.75) (see Table 2).Examination of the distribution of the A and G alleles of the Val89Leu polymorphism in the SRD5A2 gene showed that the presence of the unfavorable G allele had a predisposing value to the development of RPJ by 1.8-fold compared to the control group (48.4% vs. 34.8%, χ2=3.9; p =0.05; OR=1.8; 95%CI:1.0-3.1). At the same time, the wild-type A allele was a marker of resistance of RPV relative to the reference (51.6% vs. 65.2%, χ2=3.9; p =0.05; OR=0.6; 95%CI:0.32-1.0) (see Table 3).Table 3. Carriage of alleles and genotypes of Val89Leu polymorphism in SRD5A2 gene in the main group of patients with cancer and controls
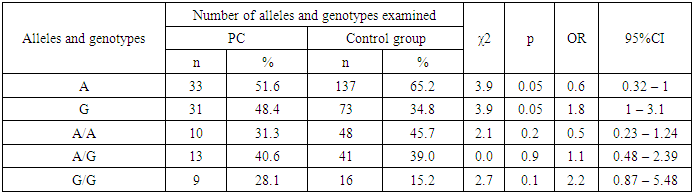 |
| |
|
However, there was a slight difference between the groups in terms of the frequency of genotypes in patients with RPF - the A/A genotype was found less frequently in the group of patients with RPF than in the control group, while the heterozygous A/G genotype was detected more frequently. It should be noted that in the group of patients with RPZ among all 32 genotypes A/A genotype was registered in 10 out of 32 cases, 31.3% and heterozygous A/G genotype in 13 out of 32 cases (40.6%), while in the control group the frequency of occurrence of these genotypes were 45.7% and 39.0% respectively. When we compared the frequency distribution of the A/A and A/G genotypes of the Val89Leu polymorphism in the SRD5A2 gene in the group of patients with RPJ and the control group, no statistically significant difference was found (χ2=2.1; p=0.2; OR=0.5; 95%CI:0.23-1.24 and χ2=0.0; p=0.9; OR=1.1; 95%CI:0.48-2.39).If we look at the rates of the group of patients with RPJ and conventionally healthy individuals, we notice that the mutant G/G genotype is less frequent in both groups, 28.1% and 15.2%, respectively.Despite insignificant differences in the percentage of occurrence, the presence of the unfavorable G/G genotype of the Val89Leu polymorphism in the SRD5A2 gene in the group of patients showed a trend in the risk of developing cancer (2.2-fold) compared with the control group (χ2=2.7; p=0.1; OR=2.2; 95%CI:0.87-5.48) (see Table 3).When analyzing the results obtained in the sample of patients with DPPD, reliable differences in the frequency of alleles were revealed in comparison with patients with cancer. Thus, the major allele A was found with χ2=5.8; p=0.03; OR=2.1; 95%CI:1.15-3.78. In turn, the minor G allele was less frequent in the group of patients with DGPH compared to those with RPH (χ2=5.8; p=0.03; OR=0.5; 95%CI:0.26-0.87), and the differences reached true significance (see Table 4).Table 4. Carriage of alleles and genotypes of Val89Leu polymorphism in SRD5A2 gene in the group of patients with BPH and PC
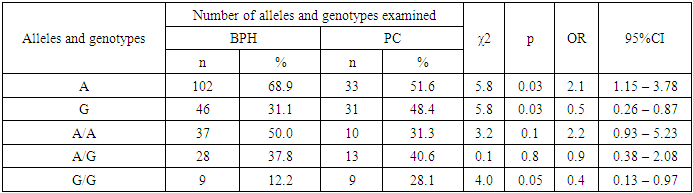 |
| |
|
The frequency of the A/A genotype above the indicated genetic polymorphism was not significantly higher in patients with DGPD, which was found in 50.0% of cases, whereas in the group of patients with RPJ, this genotype was detected in 31.3% of cases, respectively, with OR=2.2 (95%CI:0.93-5.23): and χ2=3.2 (p=0.1).The comparative analysis of the G/G genotype of the Val89Leu polymorphism in the SRD5A2 gene revealed significant differences between the groups of patients with DPPD and AD. The frequency of G/G genotype was 12.2% in patients with DGPJ and 28.1% in patients with RPJ, which demonstrated statistical significance (χ²=4.0; p=0.05; OR=0.4; 95%CI:0.13-0.97). These results indicate a possible impact of the Val89Leu polymorphism on the risk of developing AD, which emphasizes its potential role in the pathogenesis of this disease. Analysis of the frequency of the heterozygous A/G genotype showed that its prevalence among patients with DGPJ was 37.8%, whereas in patients with RPJ it was 40.6%. However, the differences in this group (χ²=0.1; p=0.8; OR=0.9; 95%CI:0.38-2.08) did not reach statistical significance and do not allow us to draw conclusions about a significant effect of this genotype on the risk of developing RPV compared to that of AD (see Table 4). Thus, the molecular genetic studies revealed that the heterozygous A/G genotype of the G2014A/Thr594Thr polymorphism was also detected with a higher frequency in the group of patients with relapse (4.1% compared to 1.9% in the control group). Although the differences also did not reach the level of threshold significance, there was a weak trend towards an increased risk of developing BPH (2.2-fold) compared to the control group (χ²=0.7; p=0.4; OR=2.2; 95%CI:0.37-12.82).In addition, a difference in the incidence of heterozygous A/G genotype of the ESR1 marker was observed in patients with AD, with a 3.4-fold increased risk of developing the disease compared to controls (6.3% vs. 1.9%, χ²=1.6; p=0.3; OR=3.4; 95%CI:0.72-16.02). These data, although not reaching statistical significance, suggest a possible influence of genetic predisposition on the risk of RPV in A/G genotype carriers. Further studies are needed to confirm this relationship and to identify potential mechanisms for the influence of ESR1 polymorphism on the pathogenesis of RPJ.In summary, the data obtained show a trend toward an increased risk of both ADHD and AD in carriers of heterozygous genotype A/G of the G2014A/Thr594Thr polymorphism in the ESR1 gene, although statistical significance has not yet been achieved. Further studies with a larger sample will help to detail the effect of this polymorphism and possibly use it as a marker to assess the risk of prostate disease.The results of the analysis demonstrate that the Arg allele and heterozygous Pro/Arg genotype of the polymorphic marker Pro/Arg in the TP53 gene can be considered as potential risk markers for the development of BPD. In particular, mutant Arg allele and heterozygous Pro/Arg genotype were detected 1.9 and 1.8 times more often in patients with BPH than in controls (χ²=3.2, p=0.10, 95% CI: 0.94-3.99, OR=1.9; χ²=2.1, p=0.20, 95%CI:0.82-3.92, OR=1.8). These data suggest that carriers of the Arg allele and the Pro/Arg genotype have an increased risk of developing BPH, even though statistical significance of the results has not yet been achieved.When comparing allele frequencies in patients with AD and practically healthy individuals, statistically significant differences were revealed. Thus, the unfavorable Arg allele was detected significantly more frequently in patients with RPF (χ²=8.3, p=0.01, 95%CI:1.46-7.16, OR=3.2), indicating an increased risk of developing RPF in the presence of this allele. The Pro/Arg heterozygous genotype was also more frequent in RPF patients compared to controls (25.0% vs. 13.3%; χ²=2.5, p=0.2, 95%CI:0.83-5.68, OR=2.2). Although the differences in the frequency of this genotype did not reach statistical significance, there was a trend towards an increased risk of RPF in Pro/Arg carriers (2.2-fold) compared to the control group.It should be noted that the Pro/Pro genotype and the Pro allele, on the contrary, may have protective properties, reducing the risk of developing cancer. This effect is supported by statistically significant results: the Pro/Pro genotype was associated with a reduced risk of RPV (χ²=5.4, p=0.03, 95%CI:0.14-0.84, OR=0.3), and the Pro allele also showed a protective effect (χ²=8.3, p=0.01, 95%CI:0.14-0.69, OR=0.3).Thus, these studies suggest a potential role for the Arg allele and the heterozygous Pro/Arg genotype as markers of increased risk for the development of BPH and AD. In parallel, the protective role of the Pro allele and the Pro/Pro genotype in relation to AD is confirmed by significant statistics, which allows us to consider them as potential predictors of reduced risk of the disease. Further studies with a larger sample will help to clarify these relationships and assess their clinical significance.In the course of this study, it was found that mutant allele G of the 34C polymorphism in the CYP17A1 gene may be one of the main causes of RPD and contributes significantly to the formation of genetic predisposition to the risk of developing the above pathology. The presence of mutant allele G of polymorphic locus 34C in CYP17A1 gene increases the risk of cancer development 2.3 times in comparison group (42.2% vs. 23.8% at χ2=8.2, p=0.01, 95%CI:1.31-4.17, OR=2.3).The analysis of the frequency of occurrence of the studied polymorphism of the genetic marker CYP17A1 revealed that the ancestral allele A and wild genotype A/A were highly significant (p=0.001) in comparison with conditionally healthy individuals (57.8% vs. 76.2% and 37.5% vs. 61.0%) (see Table 4). Moreover, the presence of the favorable A allele and homozygous A/A genotype reduced the risk of developing this pathology (χ2=8.2, p=0.01, 95%CI:0.24-0.77, OR=0.4 and χ2=5.5, p=0.03, 95%CI:0.17-0.86, OR=0.4). The mutant G/G genotype (21.9% vs. 8.6%) of the studied polymorphic locus showed the highest level of certainty, which according to OR=3.0 was registered as predisposing genotype with the highest value (95%CI:1.05-8.5) and with a high level of certainty (χ2=4.2 p=0.05) increased the risk of developing RPV by 3.0 times compared to responders.Despite the non-significant difference in the frequency distribution of heterozygous genotype A/G and mutant genotype G/G of polymorphic marker 34C in CYP17A1 gene between the compared groups, a weak tendency to the risk of developing LDCT was found and in the presence of these genotypes the risk of LDCT formation was 1. 4 and 1.9 times higher relative to the reference (at χ2=1.1, p=0.4, 95%CI:0.74-2.6, OR=1.4 and at χ2=1.7, p=0.2, 95%CI:0.74-4.7, OR=1.9).The study of the frequency of alleles and genotypes of Val89Leu polymorphism in SRD5A2 gene in patients with BPH and in conventionally healthy individuals revealed insignificant differences.Examination of the distribution of A and G alleles of the Val89Leu polymorphism in the SRD5A2 gene showed that the presence of the unfavorable G allele had a predisposing significance to the development of AD by 1.8 times compared to the control group (48.4% vs. 34.8%, χ2=3.9; p =0.05; OR=1.8; 95%CI:1.0-3.1). However, the wild-type A allele was a marker of resistance to RPV relative to the reference (51.6% vs. 65.2%, χ2=3.9; p =0.05; OR=0.6; 95%CI:0.32-1.0). Despite the slight difference in the percentage of encounters, in the presence of an unfavorable G/G genotype of the Val89Leu polymorphism in the SRD5A2 gene, there was a trend in the risk of developing RPJ (2.2-fold) in the patient group compared to the control group (χ2=2.7; p=0.1; OR=2.2; 95%CI:0.87-5.48).The comparative analysis of the G/G genotype of the Val89Leu polymorphism in the SRD5A2 gene revealed significant differences between the groups of patients with DPPD and AD. The frequency of G/G genotype was 12.2% in patients with DGPJ and 28.1% in patients with RPJ, which demonstrated statistical significance (χ²=4.0; p=0.05; OR=0.4; 95%CI:0.13-0.97). These results indicate a possible impact of the Val89Leu polymorphism on the risk of developing AD, which emphasizes its potential role in the pathogenesis of this disease.
2. Conclusions
Thus, the results of the current study emphasize the importance of genetic factors in the pathogenesis of prostate diseases. Understanding the molecular mechanisms underlying these associations may lead to the development of new strategies for the prevention and treatment of BPH and AD. It is important to further investigate the interactions between genetic polymorphisms and other risk factors such as age, family history, and lifestyle to create a more complete picture of the complex etiology of these diseases.
References
| [1] | Alcaraz A., Carballido-Rodrigues J. Quality of Life in patients with lower urinary tract symptoms associated with BPH: change over time in real-life practice according to treatment- the Qualiprost study // Int Urol Nephrol. 2016. May. 48(5). Pp. 645-56. https://www.ncbi.nlm.nih.gov/pubmed/26810324. |
| [2] | Joniau S., Briganti A., Gontero P. et al. Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study // Eur Urol. −2015. −Vol. 67(1). −pp. 157–164. |
| [3] | Kim E.H. J.A. Brockman, Andriole G.L. The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia // Asian J. Urol. – 2018. – Vol. 5, Iss. 1. – P. 28-32. |
| [4] | Lim K.B. Epidemiology of clinical benign prostatic hyperplasia // Asian J Urol. 2017. Jul. 4(3). pp. 148-151. https://www.ncbi.nlm.nih.gov/pubmed/29264223. |
| [5] | Rawla P. Epidemiology of Prostate Cancer/ P. Rawla // World J Oncol. − 2019. − Vol. 10(2). − pp. 63–89. |
| [6] | Shih H.J., Huang C.J., Lin J.A. et al. Hyperlipidemia is associated with an increased risk of clinical benign prostatic hyperplasia // Prostate. – 2018. – Vol. 78, Iss. 2. – P. 113-120. |
| [7] | Sissung T.M., Price D.K., Del Re M. et al. (2014): Genetic variation: effect on prostate cancer // Biochim. Biophys. Acta 1846, 446–456. |
| [8] | Sivonova M. K., Jurečekova J., Tatarkova Z. et al. The role of CYP17A1 in prostate cancer development: structure, function, mechanism of action, genetic variations and its inhibition // Gen. Physiol. Biophys. (2017), 36, 487–499. doi: 10.4149/gpb_2017024. |
| [9] | Vuichoud C., Loughlin K.R. Benign Prostatic Hyperplasia: epidemiology, economics and evaluation // Can J Urol. 2015. 22 (Suppl 1). Pp: 1-6. |
| [10] | Wang G. et al. Genetics and biology of prostate cancer // Genes Dev. 2018. Vol. 32, № 17-18. P. 1105–1140. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


