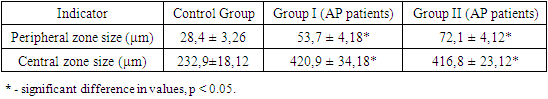-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 987-992
doi:10.5923/j.ajmms.20251504.29
Received: Mar. 10, 2025; Accepted: Apr. 1, 2025; Published: Apr. 10, 2025

Diagnostic of Aggressive Paradontitis: Pathogenetic and Diagnostic Aspects
Gaibullaev Elbek Azizbekovich1, Rizaev Jasur Alimjanovich2, Akramova Nozima Akramovna1, Esimbayeva Saule Serikovna3, Khazratov Alisher Isamiddinovich4
1Ph.D. EMU University, Tashkent, Uzbekistan
2Doctor of Medical Sciences, Professor, Samarkand State Medical University Samarkand, Uzbekistan
3Doctor of Medical Sciences, Professor, Kazakh National Medical University named after S.D. Asfendiyarov, Almaty, Kazakhstan
4Ph.D., Associate Professor, Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

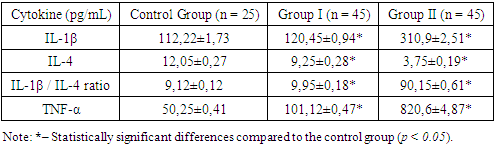
Aggressive periodontitis (AP) is a severe form of periodontal disease characterized by rapid tissue loss and significant inflammatory response. Immune mechanisms play a crucial role in the pathogenesis of AP, particularly through the activity of cytokines — mediators of inflammation that regulate the immune response and influence the progression of the disease. Studying the cytokine profile provides a deeper understanding of the pathogenesis of AP and aids in developing effective approaches for its diagnosis and treatment. This study aimed to investigate the levels of cytokines IL-1β, IL-4, and TNF-α in the gingival crevicular fluid of patients with different forms of AP. Also in this study, a comparative analysis of the salivary pattern in patients with aggressive periodontitis and clinically healthy individuals was conducted. to evaluate the clinical and radiological features of the periodontal tissue condition in patients with rapidly progressive periodontitis.
Keywords: Aggressive periodontitis, Cytokine profile, Gingival crevicular fluid, Inflammation mediators, Periodontal pockets, Clinical diagnosis, Orthopantomogram, Saliva, Dehydration
Cite this paper: Gaibullaev Elbek Azizbekovich, Rizaev Jasur Alimjanovich, Akramova Nozima Akramovna, Esimbayeva Saule Serikovna, Khazratov Alisher Isamiddinovich, Diagnostic of Aggressive Paradontitis: Pathogenetic and Diagnostic Aspects, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 987-992. doi: 10.5923/j.ajmms.20251504.29.
1. Introduction
- The search for and development of new methods for diagnosing aggressive periodontitis (AP) remains a pressing issue in modern periodontology. The contemporary literature describes numerous studies aimed not only at identifying methods of early diagnosis but also at developing approaches with high diagnostic accuracy, economic feasibility, and reproducibility [1]. The method of light microscopy of oral cavity secretions (OCS) is widely used in cases of chronic diseases. A vivid example of such an evaluation in chronic, sluggish pathologies can be seen in studies by Shabalin V.N., published between 2011 and 2018, which describe the biophysical mechanisms of solid-phase structure formation in biological fluids of the body [6,7]. In 2023, the journal "Current Issues in Medicine" published a scientific study by Solomatina N.N., which assessed facies in chronic periodontitis. The author concluded that, for generalized chronic periodontitis, a statistically significant diagnostic microscopic criterion is the marginal line of saliva pigmentation [5].It is known that the integrity of structures in the oral cavity, visible to the naked eye, depends on molecularly-oriented interrelations formed between the somatic state of the body and all structural elements of the oral cavity [2,4]. Changes in the morphological characteristics of any biological fluid, including OCS, occur in response to functional disturbances in homeostasis. In our view, this leads to pathological changes that serve as diagnostic criteria for assessing the severity of pathological processes in the oral cavity for dental practitioners [5].At present, both globally and in Uzbekistan, the number of inflammatory periodontal diseases is increasing, particularly in cases of AP [3,10,11]. The pathological process is increasingly observed starting from the age of 14 and beyond, affecting the most socially active segments of the population [11]. This fact compels researchers to seek relevant approaches for the early diagnosis of AP, when the disease still does not pose a risk of progression and does not lead to social or psychological discomfort.An imbalance in cytokines, associated with excessive production of pro-inflammatory cytokines (e.g., interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α)) and reduced levels of anti-inflammatory mediators (such as interleukin-4 (IL-4)), contributes to the progression of destructive processes in the periodontium [1,7,8]. Previous studies show that elevated levels of IL-1β and TNF-α lead to osteoclast activation [2], enhanced bone tissue destruction, and increased inflammatory activity [8,10]. Simultaneously, reduced IL-4 levels disrupt regulatory mechanisms that inhibit inflammation and promote reparative processes. Therefore, studying the cytokine profile of AP patients not only reveals the key mechanisms of the disease but also facilitates the development of individualized diagnostic and therapeutic approaches [13].Despite numerous studies, there is still insufficient data on the specific changes in cytokine profiles depending on the form of AP (localized or generalized), as well as the dynamics of these changes under therapeutic interventions [10]. The analysis of gingival crevicular fluid, as a localized biological material, represents an informative and minimally invasive method for assessing the inflammatory status of the periodontium. It allows tracking both acute and chronic changes in tissues [1]. Identifying key inflammatory mediators will enable the development of personalized strategies aimed not only at detecting the disease in the absence of pronounced clinical symptoms but also at preventing the generalization of the process and its recurrence [4,7].The aim of this study is to examine the morphological, clinical, and radiological characteristics of periodontal tissue conditions in patients with aggressive periodontitis, as well as to evaluate and analyze the levels of key pro-inflammatory and anti-inflammatory cytokines in the gingival crevicular fluid of patients with AP.
2. Materials and Methods
- In accordance with the design of this study, material from 90 patients with a clinical diagnosis of aggressive periodontitis (AP) was analyzed. Based on the inclusion criteria, the average age of patients in the AP group was 18.1 ± 0.11 years. Patients included in the study had no concomitant somatic pathology or harmful habits (e.g., smoking). All AP patients were divided into two groups: Group I included 45 patients with mild localized AP, while Group II comprised 45 patients with a generalized form of AP. The control group included 25 patients aged 18–28 years (mean age: 18.9 ± 0.28 years) with a sanitized oral cavity.During the clinical examination, the condition of the hard dental tissues, occlusion, and caries presence were assessed, including its prevalence and intensity. Samples of oral cavity secretions (OCS) were collected following standardized procedures in the morning, on an empty stomach, in a well-lit room. A critical condition for the study was the absence of psychoemotional stress and intense physical activity (participants were advised not to visit the gym on the day of sample collection). Before the procedure, participants rinsed their mouths with distilled water (100 mL for 3–5 minutes).To evaluate the intrinsic crystallization activity, 1–2 drops of secretion were placed on a clean (degreased), sterile microscope slide using a semi-automatic dispenser. The slides were dried under standard room conditions (air temperature: 24°C, humidity: 65%) for 24 hours. Microscopic examination of the samples began no earlier than 24–25 hours after the start of the study. Light microscopy was performed using a light microscope with transmitted light and a built-in digital camera. The analysis followed the list of markers of oral pathology proposed by Shatokhina S.N. in 2013 [8]. Visualization and measurement of the main zones were performed using the "ScreenMeter" image morphometry software. For detailed zone displacement analysis, a reference template was overlaid on the images in Adobe Photoshop.Periodontal probing was performed in AP patients, with pocket depths >5 mm at the deepest point. Cytokine levels were determined by collecting gingival crevicular fluid (GCF). The sampling site was pre-cleaned of biofilms and soft dental plaque and isolated from saliva using cotton rolls. The GCF was absorbed using filter paper until fully saturated and then placed in an "Eppendorf" tube containing sterile 0.9% sodium chloride solution and frozen at –20°C. Cytokines (IL-1β, IL-4, TNF-α) were analyzed using "Vector-Best" test systems (Novosibirsk, Russia) following the manufacturer’s instructions.All participants included in the study were assessed for oral hygiene index (OHI-S) [5], periodontal tissue inflammation index (PMA), periodontal index (PI), and Muhlemann bleeding index. Radiological examinations were performed on all patients to assess the condition of the alveolar bone, including bone pockets, furcation defects, and other destructive changes.Statistical analysis of the results was performed using Statistica 10.0 software, employing methods of variation statistics and Student's t-test for normally distributed data. Results were presented as arithmetic means ± standard error (M ± m), with significance set at p < 0.05.The study was conducted in accordance with international ethical guidelines for biomedical research involving human subjects.
3. Results and Discussion
- The examined patients reported a range of clinical complaints: 100% of patients noted halitosis, and bleeding was observed during routine daily oral hygiene procedures. Clinical examination revealed pathological tooth mobility and changes in tooth position. The clinical findings showed that 45% of patients exhibited no visible signs of microcirculatory response, such as edema or mixed hyperemia of gingival tissues. However, despite the mild clinical presentation of inflammation, patients with AP had an average periodontal pocket depth of 7.5 mm, with some cases exceeding 8.5 mm. In Group II, 36% of patients exhibited grade II–III tooth mobility. No purulent exudate was detected.Orthopantomographic data from Group II patients revealed massive generalized destruction of alveolar bone, including both vertical and horizontal types. Bone loss reached up to 2/3 of tooth root length, with grade III furcation defects and osteoporosis. In Group I, bone destruction was also observed but was less pronounced.
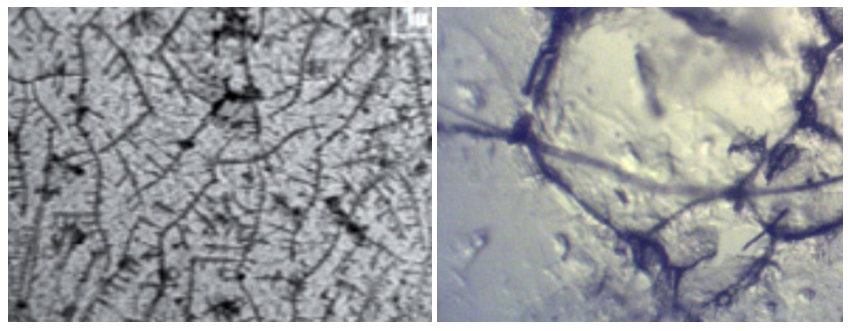
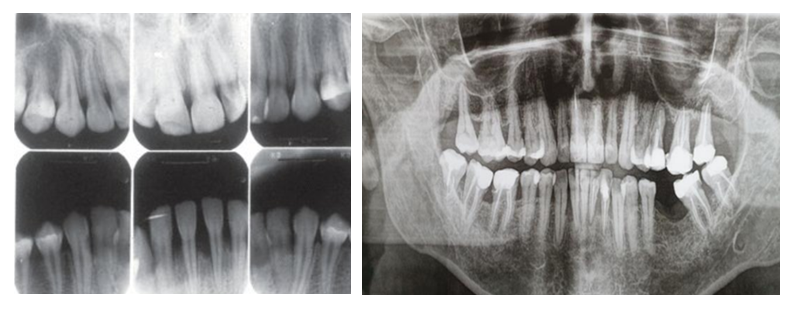 | Figure 1. Radiographs of patients from Group I and Group II |
|
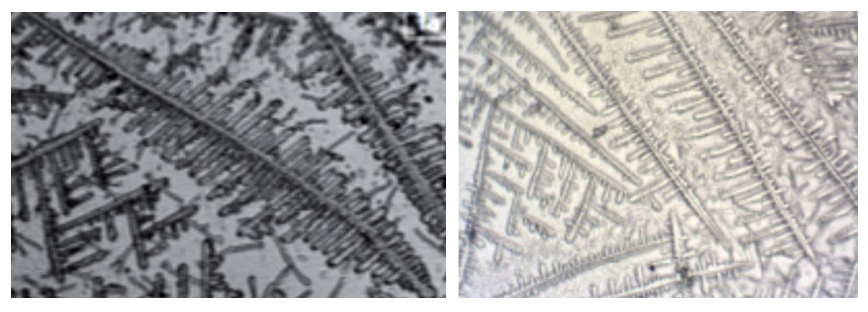 | Figure 2. Light microscopy image of mixed saliva from the control group |
 | Figure 3. Light microscopy of mixed saliva in patients with aggressive periodontitis |
|
|
4. Conclusions
- 1. The evaluation of quantitative indicators of mixed saliva crystallization demonstrated a statistically significant imbalance in patients with the generalized form of aggressive periodontitis.2. Quantitative diagnosis of mixed saliva crystallization serves as a clinical and diagnostic marker for the severity of pathological processes in the oral cavity in aggressive periodontitis.3. Patients with aggressive periodontitis exhibit a significant increase in pro-inflammatory cytokine levels (IL-1β, TNF-α) and a decrease in the anti-inflammatory cytokine IL-4 in gingival crevicular fluid.4. The IL-1β/IL-4 ratio can be used as a marker of inflammatory process activity.5. Cytokine profile assessment is crucial for diagnosing and monitoring the prognostic significance of aggressive periodontitis.6. The generalized form of aggressive periodontitis is characterized by significant bone tissue destruction with relatively mild inflammatory signs, indicating a complex pathogenic mechanism of the disease.7. The OHI-S index values indicate a low level of oral hygiene among patients, which may highlight the etiological significance of the microbial factor in the disease's pathogenesis.8. For accurate diagnosis and a personalized approach to managing patients with aggressive periodontitis, it is essential to correlate the results of clinical examination with radiological findings of bone structure changes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML