Turgunov A. I.
Andijan State Medical Institute, Andijan, Uzbekistan
Correspondence to: Turgunov A. I., Andijan State Medical Institute, Andijan, Uzbekistan.
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Prosthetic valve obstruction (PVO) is a rare but feared complication of mechanical valve replacement. Diagnostic evaluation should focus on differentiating prosthetic valve thrombosis (PVT) from pannus formation, as their treatment options differ. History of sub-optimal anti-coagulation and post-op time course to development of PVO are useful clinical characteristics in differentiating thrombus from pannus formation. Treatment of PVT is influenced by the patient’s symptoms, valve location, degree of obstruction and thrombus size and may include thrombolysis or surgical intervention. Alternatively, pannus formation requires surgical intervention. The purpose of this article is to review the pathophysiology, epidemiology, diagnostic approach and treatment options for aortic and mitral valve PVO.
Keywords:
Prosthetic valve thrombosis, Pannus overgrowth, Mitral valve replacement, Prosthetic valve obstruction, Echocardiography
Cite this paper: Turgunov A. I., Obstruction of Mechanical Heart Valves, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 887-892. doi: 10.5923/j.ajmms.20251504.08.
1. Introduction
The cause of heart valve obstruction may be prosthetic thrombosis, pannus, or a combination of both. The frequency of thisго serious complication, according to various authors, ranges from 0.15 to 25% [1,2,4,7,8,10].Prosthetic thrombosis occurs more frequently in the mitral position (18-40%) than in the aortic one (0-15%) [2,4,5,9,11,13,15]. However, other authors believe that the process of thrombosis is more susceptible to prostheses located in the tricuspid position [3,6,14].There is no consensus on the degree to which prostheses of various types and designs are susceptible to thrombosis, although the latest generation of valves (Medinzh-2, St.. Jude Medical, Carbomedics, Sorin, etc.) are characterized by greater thrombosis resistance [5,8,13,17].It is believed that the main cause of artificial valve thrombosis is inadequate anticoagulant therapy. At the first stages of valve replacement surgery, without the general concepts of anticoagulant therapy, prosthetic thrombosis and thromboembolic complications reached 50-75% [2,4,16]. All this has forced cardiologists and surgeons to develop and implement blood coagulation inhibitors. Currently, the percentage of these complications has significantly decreased during anticoagulant therapy and ranges from 0.5 to 4.5% patienta/ year [2,3,6,7,8,15].At the present stage of cardiac surgery, most patients have a long life span with prosthetic heart valves, and follow-up periods for these patients are being extended. A number of authors note an increase in the incidence of pannus (excessive growth of the pseudoendocardium) up to 25% in the long term, which can be a provoking factor of prosthetic thrombosis due to its stenosis, or independently disrupt its function [8,10]. In this regard, the authors conclude that the term "prosthetic thrombosis" is not equivalent to the term "prosthetic obstruction" and suggest using the latter until intraoperative data are obtained.The clinical course of mechanical heart valve obstruction is variable, so proper diagnosis can be difficult. In some patients with prosthetic dysfunction, the clinical picture may be absent and the presence of prosthetic obstruction may indicate thromboembolism in the peripheral arteries. With significant occlusion of the prosthesis, a clinic of severe heart failure is observed. Thus, successful treatment of mechanical heart valve obstruction largely depends on timely and correct diagnosis, where echocardiography plays a leading role. The presence of prosthetic thrombosis is a direct indication for re-operation. In a number of studies, the authors suggest removing blood clots from the surface of the prosthesis, indicating a low hospital mortality rate compared to reprosthetics [2,3,4,7,16]. Others recommend changing the prosthesis, as thrombosis may be related to the design features of this prosthesis [5,14,15]. There are also reports of conservative treatment of prosthetic thrombosis with urokinase and streptokinase, but the results obtained indicate that this technique is not fully effective, since prosthetic dysfunction can be caused not only by thrombosis, but also by a combination of a thrombus and a pannus.Thus, the treatment of mechanical heart valve obstruction is a challenging task of acquired heart disease surgery. Repeated interventions in this cohort of patients in most cases are performed in conditions of severe disorders of intracardiac hemodynamics. In some cases, the diagnosis of obstructive lesions of mechanical heart valves is difficult due to the unclear clinical picture. The question of the timing of re-operation is debatable. This issue is especially acute in patients without signs of heart failure, but with episodes of thromboembolism in the peripheral arteries. Until now, there is no consensus in choosing the method of surgical correction. Certain difficulties are associated with the selection and duration of anticoagulant therapy. In addition, there are no well-developed clear methods of preventing this complication.
2. Materials and Methods
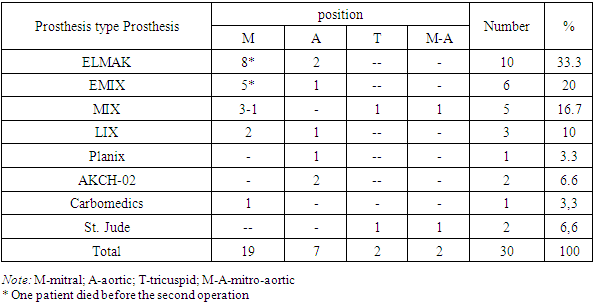
During the period from 2012 to23 2023, the Department of Adult Cardiac Surgery of the Academician Vakhidov National Medical Center and the Department of Cardiac Surgery of the Clinic of the Andijan State Medical Institute included 30 patients with detected obstruction of a mechanical prosthetic heart valve (Table 1). The patients included 18 men and 12 women, ranging in age from 16 to 66 years, with an average age of 42.2±14.2 years. According to intraoperative and autopsy data, prosthetic thrombosis occurred in 18 (60%) patients, in 7 (23.3%) – a combination of thrombosis and pannus, in 5 (16.7%) – isolated pannus. Table 1. Type and position of obstructed prostheses
 |
| |
|
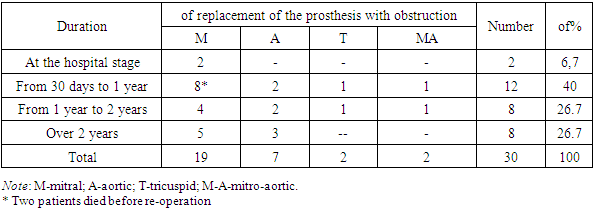
Obstruction of the mitral prosthesis was observed in 19 (63.3%) patients, aortic – in 9 (30%) and tricuspid – in 2 (6.7%).Repeated surgery was performed in 28 patients. The median time from primary surgery to obstruction was 36 weeks (3 days to 15 years). In 2 (6.7%) patients, mitral prosthesis thrombosis occurred at the hospital stage. The time from the onset of symptoms to surgery ranged from 1 to 30 days in 18 (60%) patients, from 30 days to a year – in 10 (33.3%), in 2 (6.7%) patients, the duration of symptoms was more than a year.Table 2. Terms from the primary operation to the appearance of prosthetic obstruction (N=30)
 |
| |
|
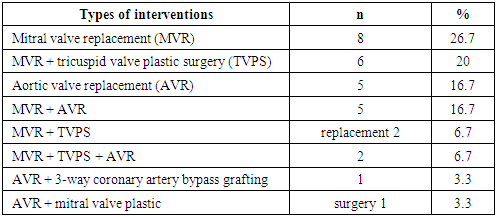
Among the operated patients, 11 (36.7%) were assigned to IIINYHA functional class IIINYHA, and 17 (56.7%) were assigned to IVfunctional class IV. Grade IIA circulatory insufficiency was observed in 17 (56.7%) patients, grade IIB – in 11 (36.7%). Two patients (6.7%) died before the second operation, due to a critical hemodynamic disorder (pulmonary edema, hypotension).The main etiological factor in the development of heart disease was the rheumatic process – in 22 (73.3%) patients. In 5 (16.7%) cases, the cause was infectious endocarditis and in 2 (6.7%) – congenital bicuspid aortic valve. In our study, 2 (6.7%) patients underwent surgery for the third time - they underwent closed mitral commissurotomy prior to valve replacement. In addition, concomitant pathology was corrected during the primary operation (Table 3).Table 3. Types of interventions at the primary surgery
 |
| |
|
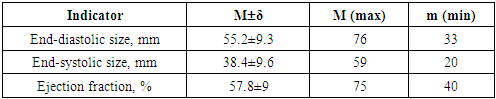
Data on the effectiveness of anticoagulant therapy were available in 22 (73.3%) patients with prosthetic obstruction. Inadequate anticoagulant support was observed in 14 (63.6%) of them, while in 8 cases there were significant interruptions in the use of anticoagulants.Sinus rhythm among the operated patients was observed in 8 (47%) patients, the rest - atrial fibrillation, which developed on average in 4.75±1.3 years before going to the clinic (1 to 13 years). According to X-ray data, venous congestion in the small circle of blood circulation was present in 76.7% of patients, signs of pulmonary hypertension-in 16.7%, and pulmonary edema - in 6.7%.According to transthoracic echocardiography, the size of the left atrium in the study group was from 40 to 72 mm and averaged 58.2±2.8 mm (Table 4).Table 4. Preoperative echocardiographic parameters (n=28)
 |
| |
|
The most frequent signs of dysfunction were the presence of additional echo signals in 22 (73.3%) patients and a decrease in the amplitude of movement of the locking element – in 12 (40%). Doppler echocardiography showed an increase in the mean diastolic gradient on the prostheses: on the mitral prosthesis – 15.3 ± 3.5 mm Hg (max – 19), on the aortic prosthesis – 49 ± 2.6 mm Hg (max – 57) and on the tricuspid prosthesis-8.5 ± 0.49 mm Hg (max-9).To clarify the diagnosis, 19 (63.3%) patients underwent transesophageal echocardiography. In this study, all 19 (in 100% of cases) examined patients showed a deviation or change in the diastolic flow through the prosthesis, which spoke in favor of obstruction of the prosthesis. However, in two (6.7%) patients with mitral-aortic prostheses, transthoracic and transesophageal echocardiography revealed thrombosis of the mitral prosthesis only, while the operation revealed thrombosis of the aortic prosthesis as well.8 patients (26.7%) underwent coronary angiography. Hemodynamically significant stenoses were found in 1 (3.3%) patient, and another patient had venous bypass occlusion to the right coronary artery. Hemodynamically insignificant stenoses (less than 55%) were detected in 2 patients, which was 6.7%.Surgical treatment of mechanical heart valve obstructionDepending on the severity of the condition and the timing of surgical intervention, patients were divided into two groups (Table 5). Group 1 included patients in extremely serious condition (IVNYHA FC IVNYHA), in which surgical intervention was performed no later than 4 days after admission for urgent indications (n=16). Group 2 included patients who managed to improve their condition by conservative methods and underwent surgery more than 4 days after admission (n=12).Table 5. The nature of reoperations depending on the term of their implementation
 |
| |
|
As can be seen from the table, more than half of the patients (57.1%) underwent urgent surgery.Repeated surgery was performed in all patients with cardiopulmonary bypass, pharmaco-cold cardioplegia (Custodiol), and moderate hypothermia of 28°C. The average time of cardiopulmonary bypass was 152±40.2 minutes, and aortic compression was 107.7±44.6 minutes. Femoral artery cannulation was performed in 6 (21.4%) patients.During surgery, in most cases, the cause of obstruction was macroscopically thrombosis – in 17 (60.7%) patients, thrombosis in combination with pannus – in 6 (21.4%) (Figure 1), isolated pannus – in 5 (17.8%).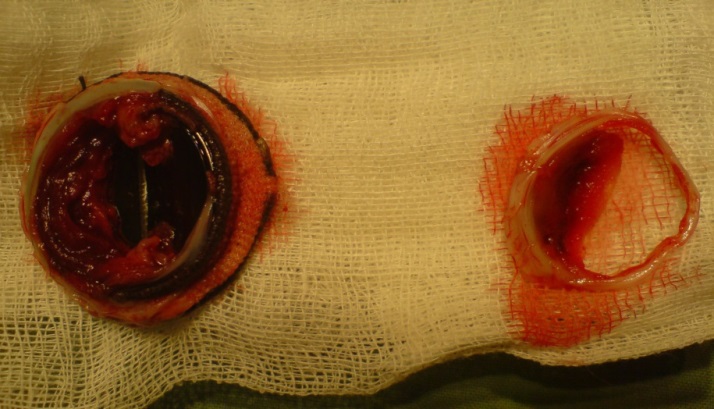 | Figure 1. Macroscopically thrombosis |
Surgical treatment consisted of replacing one or two prostheses (Table 6). Two (7.1%) patients from group 1 underwent thrombectomy and pannus excision. When declotting (cleaning the prosthesis), we aimed to reduce the time of myocardial anoxia in initially severe patients (IVNYHA functional class IVNYHA).Table 6. Nature of repeated interventions performed (n=28)
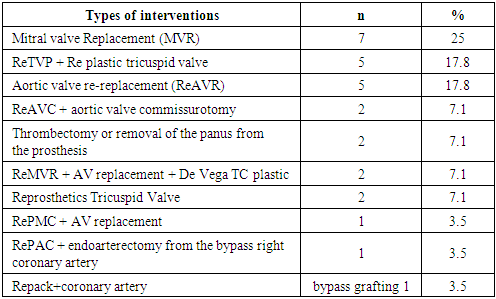 |
| |
|
In two (7.1%) patients, tricuspid valve replacement was performed on a fibrillating heart. When replacing the prosthesis, biological valves (EpicTM Supra Valve No. -29) were used. The operation on the fibrillating heart was carried out without technical difficulties, the rhythm was restored after one discharge of the electrodifibrillator. In two cases, subvalvular structures were not preserved during the primary mitral valve replacement surgery.
3. Immediate and Long-Term Results
Good and satisfactory results were obtained in 25 (83.3%) patients. Good results were observed in I9 (56.2%) patients in group III, and in 10 (83.3%) patients in group II. Patients showed a significant improvement in their condition, no preoperative complaints disappeared, and the left ventricular ejection fraction was more than 55%. Satisfactory results were observed in IGroup I in 4 (25%) patients, in IIgroup II – in 2 (16.7%). Subjectively, these patients felt an improvement in their condition, there were no signs of decompensation in the large and small circulatory circles. However, the left ventricular ejection fraction was less than 50%. Echocardiographic parameters after surgery are presented in Table 7.Table 7. Echocardiographic parameters after surgery (n=25)
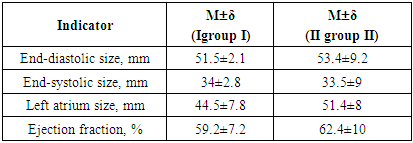 |
| |
|
Overall hospital mortality was 16.7% (5 patients). Without repeated intervention, 2 (6.7%) patients with prosthetic thrombosis died, while one in an extremely serious condition died a few hours after admission. The cause of death was progressive myocardial heart failure. One patient developed mitral prosthetic thrombosis three weeks after the initial intervention. The patient died of acute cardiovascular failure. In both cases, the diagnosis was confirmed at autopsy.Of the 14 patients with severe cardiac decompensation who underwent emergency surgery in the next 4 days (Group 1), 3 (18.7%) died.Of the 12 patients operated on at a later time (Group 2), there were no deaths. No fatal outcomes were observed in the group of patients with IIIfunctional class III. All the deceased patients belonged to the IV functional class and had decompensation in the large circle of blood circulation. Univariate analysis showed that the only significant risk factor for hospital mortality was IVfunctional class IV (NYHA) and decompensation in the large circulatory circle (p<0.05).Non-fatal complications that developed in the postoperative period are presented in Table 8.Table 8. Non-fatal complications in the postoperative period (N=25)
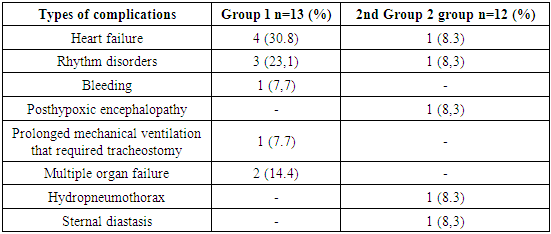 |
| |
|
As can be seen from the table, heart failure ranked first among the complications (39.1%). This group included patients who had a "low ejection" syndrome and required significant cardiotonic support in the postoperative period (epinephrine more than 0.07 mcg / kg / min, dopmin more than 8 mcg / kg / min, dobutrex 10 mcg / kg / min). The dose of cardiotonics was reduced gradually. On average, hemodynamics in these patients stabilized on 4-5 days after surgery, which allowed us to cancel catecholamines.The second place among complications was occupied by rhythm disorders (31.4%). In most cases, these were transient rhythm disturbances in the form of atrioventricular block of the 1st degree, which were stopped on the 7th-8th day after surgery. In one patient, rhythm disturbances were manifested in the form of paroxysms of atrial fibrillation, which were stopped by medication (cordarone).One patient underwent surgery on the 18th day after surgery due to sternal diastasis. During the operation, eruption of 2 dacron sutures in the area of the sternum handle was noted. The sternum is sewn with metal threads. The patient was discharged in satisfactory condition two weeks after repeated sternum suturing.25 patients were discharged from the hospital: 13 from group 1 and 12 from group 2. Long-term results were studied in terms from 6 months to 8 years based on the data of questionnaires and examinations in our clinic. One patient from the second group was lost from observation. The completeness of observation was 96%.In the long-term period, two (15.4%) patients in group 1 died. One patient died 1.5 months after mitral valve replacement due to the development of early prosthetic endocarditis. The second patient died 2 months after re-operation in the neurological department from pulmonary heart failure, where she was transferred due to neurological disorders after the operation.In the second group, 1 (8.3%) patient died from an ischemic stroke 2 years after surgery.Good results in the long-term period were observed in 7 (33.3%) patients. These patients showed significant improvement in their condition, no preoperative complaints, left ventricular ejection fraction was equal to or greater than 55%, NYHA and they met NYHA IFunctional Class I. Satisfactory results were obtained in 13 (61.9%) patients. These patients showed an improvement in their condition compared to preoperative, there were no signs of decompensation in the large and small circulatory circles, the left ventricular ejection fraction was at least 50%, and according to NYHA they met IIfunctional class II. An unsatisfactory result was observed in one (4.7%) patient. 2 years after the operation, the patient suffered a thromboembolism in the cerebral vessels with a rapid regression of symptoms. The long-term survival rate of patients up to 8 years was 75.7±7.8%. The majority of patients (95.3%) in the long-term period after repeated intervention have a significant improvement in their condition and are in I и IIfunctional class I and II, which confirms the need for surgical treatment regardless of the severity of the condition at admission. The main aggravating factor in the long-term period after re-operation remains, first of all, thromboembolic complications, and in our case, the same dependence remains – the highest frequency of thromboembolism occurs in the first 2 years after re-operation. Repeated thrombosis of the prosthesis in the long-term period was not detected in our group of patients. However, taking into account the thromboembolic complications, we believe that anticoagulant therapy should be strengthened for patients undergoing surgery for prosthetic thrombosis, simultaneously affecting various factors of the blood coagulation system. Thus, timely diagnosis, properly selected conservative therapy and urgent surgical intervention can improve the survival rates of patients with obstructive heart valve lesions.
4. Conclusions
1. The cause of obstruction of mechanical heart valves can be thrombosis, pannus, or a combination of both. 2. In the surgical treatment of mechanical heart valve obstruction, we do not refute the possibility of declotting, but in most cases we perform reprosthetics, which gives more predictable results.3. The postoperative mortality rate was 10.7%, and the eight-year actuarial survival rate was 75.7±7.8%.
References
| [1] | Bazylev V. V., Mikulyak A. I., Shmatkov M. G., Senzhapov I. Ya., Denisov M. A. Open reprosthetics of the mechanical mitral valve with a balloon-expandable prosthesis using the ≪valve-to-valve technique≫: a clinical case. Circulatory pathology and cardiac surgery. 2024; 28(1): 19-25. DOI: 10.21688/1681-3472-2024-1-19-25. |
| [2] | Bokeria L. A., Milievskaya E. B., Kudzoeva Z. F. et al. Cardiovascular Surgery-2018. Diseases and congenital anomalies of the circulatory system. Moscow: A. N. Bakulev National Research Medical Center of Agriculture of the Ministry of Health of the Russian Federation, 2019, p. 270. ISBN: 978-5-7982-0408-3. |
| [3] | Vladimirov V. V., Kokov L. S., Kovalev A. I., Niyazov S. S., Parkhomenko M. V., Redkoborodyj A.V., Rubtsov N. V., Bikbova N. M., Muslimov R. Sh. The first experience of aortic valve replacement using the "valve in valve" technique in a patient with a biological prosthesis dysfunction. Russian Sklifosovsky Journal of Emergency Medical Care. 2021; 10(3): 582–588. https://doi.org/10.23934/2223-9022-2021-10-3-582-588. |
| [4] | Ganyukov V. I., Shloydo E. A., Tarasov R. S. et al. Transeptal transcatheter implantation of a bioprosthesis using the "valve-to-valve" technique for dysfunction of a biological prosthesis in the mitral position: the first experience. Circulatory pathology and cardiac surgery. 2020; 24(1): 94-103. https://doi.org/10.21688/1681-3472-2020-1-94-103. |
| [5] | Evseev E. P., Podolyak D. G., Lutokhina Yu. A., Osadchaya V. A., Morozova N. V., Chichkova N. V., Dombrovskaya A.V., Fomin M. A., Aidamirov Ya. A., Voropaeva V. I., Dzeranova A. N., Dzemeshkevich S. L. Prosthetics of heart valves in hypertrophic cardiomyopathy: unavoidability of the situation, efficiency and risks // Clinical and experimental surgery. Journal named after Academician B. V. Petrovsky. 2024. Т. 12, № 3. С. 104-109. DOI: https://doi.org/10.33029/2308-1198-2024-12-3-104-109. |
| [6] | Evseev E. P. et al. Surgical treatment of heart defects from right-sided mini-thoracotomy / / Cardiology and cardiovascular surgery. - 2021. - Vol. 14. - No. 1. - pp. 26-31. |
| [7] | Eliseev I. G., Lischuk A. N., Khavandeev M. L. et al. Evolution of mitral valve replacement. Bulletin of the National Medical and Surgical Center named after N. I. Pirogov 2024, vol. 19, No. 3. DOI: 10.25881/20728255_2024_19_3_118. |
| [8] | Klyshnikov K. Yu., Ovcharenko E. A., Stasev A. N., Barbarash L. S. Repeated prosthetics of heart valves: approaches and devices (literature review). Cardiovascular therapy and prevention. 2023; 22(2): 3377. doi:10.15829/1728–8800-2023-3377. |
| [9] | Kozlov B. N., Petlin K. A., Kosovskikh E. A. et al. The first experience of using a valve-containing conduit with a biological aortic valve prosthesis and the "easy change" system. Cardiology and cardiovascular surgery. 2019; 12(5): 429-32. https://doi.org/10.17116/kardio201912051429. |
| [10] | Posokhov I. N., Praskurnichy E. A., Morozova O. I. Evaluation of prosthetic valve function in modern clinical practice. Effective pharmacotherapy. 2024; 20 (26): 54–60. DOI 10.33978/2307-3586-2024-20-26-54-60. |
| [11] | Chernov I. I., Enginoev S. T., Komarov R.N., Tarasov D. G., Sinelnikov Yu. S., Marchenko A.V. et al. Three-year results of Ozaki surgery in patients 65 years and older: a multicenter study. Circulatory pathology and cardiac surgery. 2021; 25(4): 53-63. https://doi.org/10.21688/1681-3472-2021-4-53-63 |
| [12] | Shevchenko Yu. L., Tsvetkova T. V., Gudymovich V. G., Vasilashko V. I. Long-term result of mitral and aortic valve replacement with domestic ball prostheses 35 years after surgery (clinical observation with a brief literature review) // Bulletin of the National Medical and Surgical Center named after N. I. Pirogov. - 2020. - Vol. 15. - No. 3-2. - pp. 172-178. doi: 10.25881/BPNMSC.2020.46.81.030. |
| [13] | 2021 ESC/EACTS Guidelines for the management of patients with valvular heart disease. Russian Journal of Cardiology. 2022; 27(7): 5160. https://doi.org/10.15829/1560-40712022-5160. |
| [14] | Arutyunyan V., Chernov I., Komarov R., Sinelnikov Y., Kadyraliev B., Enginoev S., et al. Immediate outcomes of aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium: A multicenter study. Brazilian J Cardiovasc Surg. 2020; 35: 241-8. https://doi.org/10.21470/1678-9741-2020-0019. |
| [15] | Koziarz A, Makhdoum A, Butany J, et al. Modes of bioprosthetic valve failure: a narrative review. Curr Opin Cardiol. 2020; 35(2): 123-32. https://doi.org/10.1097/HCO.0000000000000711. |
| [16] | Nicolas Dürrleman 1, Michel Pellerin, Denis Bouchard, Yves Hébert, Raymond Cartier, Louis P Perrault, Arsène Basmadjian, Michel Carrier. Prosthetic valve thrombosis: twenty-year experience at the Montreal Heart Institute. Thorac Cardiovasc Surg. 2004 May; 127(5): 1388-92. doi: 10.1016/j.jtcvs.2003.12.013. |
| [17] | Ole DB, Lars S. Redo-TAVR: What About the Coronary Arteries? JACC Cardiovascular Interv. 2020; 13(22): 2628-30. https://doi.org/10.1016/j.jcin.2020.10.005. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML






