-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 883-886
doi:10.5923/j.ajmms.20251504.07
Received: Mar. 5, 2025; Accepted: Mar. 28, 2025; Published: Apr. 6, 2025

Experience of Successful Surgical Correction of Paraprosthetic Mitral Valve Fistula
Turgunov A. I.1, Aliev Sh. M.2, Kasimov A. L.1, Turgunov B. A.1, Mansurov Sh. Sh.1, Mamadiev X. M.1
1Andijan State Medical Institute, Andijan, Uzbekistan
2The Republican Specialized Scientific and Practical Medical Center for Surgery named after Academician V. Vakhidov, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The authors of this scientific article present a case of successful surgical correction of paraprosthetic fistula of the mitral valve. For adequate and complete elimination of paraprosthetic fistula of the mitral valve, a method of plastic surgery with a xenopericardial patch was used. This method allows to reduce intraoperative complications and the risk of re-formation of paraprosthetic fistula. To diagnose paraprosthetic fistula of the mitral valve, it is necessary to use informative and high-tech research methods (MSCT, EchoCG), which make it possible to perform timely correction of paraprosthetic fistulas. This method may be promising, but further improvement of the surgical technique is required, as well as a study of the remote results of repeated operations.
Keywords: Paraprosthetic mitral valve, Surgical correction, Heart valve diseases, MSCT, EchoCG
Cite this paper: Turgunov A. I., Aliev Sh. M., Kasimov A. L., Turgunov B. A., Mansurov Sh. Sh., Mamadiev X. M., Experience of Successful Surgical Correction of Paraprosthetic Mitral Valve Fistula, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 883-886. doi: 10.5923/j.ajmms.20251504.07.
1. Introduction
- Every year in the world valve replacement hearts is being carried out approximately 210 thousand patients. As the number of operations increases, so does the number of prosthesis dysfunctions due to valve-dependent complications. One of them is paraprosthetic fistulas.It is believed that the formation of paraprosthetic fistula non-infectious genesis in most cases, it is associated with technical errors during the initial operation. Frequency their occurrence, according to literature data, ranges from 3 to 12.5% [1]. At the same time, hospital mortality still remains quite high. high - from 11 to 25% [1]. There is no consensus regarding the dependence of the frequency of fistula formation on localization. Some authors indicate that the formation of paraprosthetic fistulas is happening more often V aortic positions (4.9%), how V mitral (2%) [2]. Other researchers have found no differences in the incidence of of this complications at sick With mitral and aortic prostheses. After mitral valve replacement (MV), fistulas most often form in the area septal cusp, due to the fact that the surgeon, fearing to involve the aortic valve cusp in the stitch, can capture less tissue. In the aortic position, the fistula is more often localized in the region of the left coronary and non-coronary cusps: the reason is the fear of involving the septal cusp of the mitral valve in the stitch. In addition, a certain role is played by the desire to avoid injuries atrioventricular node. When using MK prosthetics, it is especially dangerous areas, adjacent To external and internal commissaries, So How V these areas of the coronary arteries are maximally approached To fibrous ring. Hence, overlay deep sutures in these areas of the fibrous ring increase the risk of damage to the coronary arteries, What Maybe bring To lethal from the course on operating table. Frequency this formidable complications makes up 1-3%. State fibrous rings plays an important role role V emergence paraprosthetic fistulas. Presence of valve calcification With transition on fibrous the ring is predisposing factor for education paraprosthetic regurgitation in the postoperative period. Imposition seams on calcified areas of the fibrous ring may lead to damage to the suture material. To prevent this complication, it is necessary to carry out careful decalcification before suturing the fibrous ring. However, it should be noted that What radical decalcification may lead to damage to the fibrous rings And anatomical formations located in close proximity from him, A Also walls left ventricle (LV) with subsequent bleeding, formation aneurysms or defect interventricular partitions.Besides calcinosis fibrous rings predisposition To education a pair of prosthetic fistulas is observed in the presence of degenerative changes fabrics fi broznogo rings, characteristic For valve prolapse and Marfan syndrome, with sharp changes in valve structures, rupture of chords, weakness of the fibrous ring.The reliability of the prosthesis attachment is significantly reduced if it is incorrectly selected. size. Implantation prosthesis a smaller size causes tension on the fixing sutures, and a prosthesis that is too large injures the endocardium, leading to tears And hemorrhages - V In both cases there is a risk of fistula formation [4].Also, the cause of fistulas may be poor quality of suture material, incorrect placement of sutures or insufficient number of sutures [3].Mainly the formation of paraprosthetic fistula is happening V first months after operations [5]. TO indications For repeat operations relate detection fistulas And progression cardiac insufficiency as a result increases left sections hearts, availability hemolysis. The question of reoperation should be decided strictly individually, depending on the degree and rate of increase of signs of circulatory failure [1,2,3].Currently, in most cases, clinically significant paraprosthetic Regurgitation is eliminated surgically using the method of complete correction of the fistula, but the risk of recurrent fistula formation and complications associated with it is significantly higher than the risk of treating paraprosthetic fistulas by plastic surgery using a patch - an alternative treatment method introduced into clinical practice relatively recently. In the domestic literature, we did not find reports on the closure of paraprosthetic fistulas with a patch, in connection with which we present our own observation - a clinical case of successful closure of a paraprosthetic fistula of the mitral valve using this method.
2. Clinical Case
- We present a case of clinical observation: Patient H., 59 years old (case history No. 1789/1126), was hospitalized on June 23, 2022 Department of Cardiac Surgery of the Altai State Medical Institute on a planned basis with the diagnosis: Main: "Chronic rheumatic heart disease. Mitral heart defect. Condition after the operation of closed commissurotomy of the mitral valve (2002), prosthetics of the mitral valve with the biological valve Epic TM Supra Valve #29 (2021).Complications: CHF stage II B. FC III according to NYHA. AF paroxysmal form. Paravalvular 3rd degree regurgitation. State of acute cerebrovascular accident with left-sided hemiparesis (2021).Associated diseases: Type 2 diabetes mellitus, moderate severity in the decompensation stage. Nodular goiter.Upon admission, she complained of palpitations, shortness of breath and a feeling of lack of air, occurring with little physical exertion and at rest, thirst, loss of appetite, rapid fatigue, general weakness and decreased ability to work. It was established that the patient had been suffering for several years, was treated in a hospital and outpatient setting, and was also under the supervision of a cardiologist, cardiac surgeon, neurologist and endocrinologist at her place of residence. In recent years, the patient was treated by a neurologist and endocrinologist and was also under dispensary supervision. From the anamnestic information, the patient underwent ZMK in 2002, mitral valve replacement in 2021, thrombectomy from the left atrium. Recently, the general condition of the patient gradually began to deteriorate, and complex treatment measures did not lead to a noticeable improvement and were not crowned with success. Despite the intensive complex treatment measures, the above-mentioned subjective sensations progressively increased and did not tend to decrease. In this regard, the patient was hospitalized in the specialized department of cardiovascular surgery of the ASMI clinic. Upon admission, the general condition of the patient was assessed by us as severe due to the increase in heart failure. The patient's motor activity is reduced, the skin is pale with periodic cyanosis. Respiratory rate is 20-25 times per minute. Auscultation reveals harsh breathing in the lungs. Heart sounds are muffled and the borders of the heart are dilated. Heart rate is 143 beats / min. Pulse 125-130 beats / min. Hepatomegaly, liver + 3 cm from the edge of the costal arch. Spontaneous diuresis is 1-1.5 liters per day. A comprehensive examination was carried out in the clinic using instrumental and laboratory research methods.In transesophageal EchoCG: biological valve functions, paravalvular fistula is detected around the mitral valve A 1, A 2. Dilation of the left atrium. No left ventricular hypokinesia was detected, myocardial contractility is preserved. No separation of pericardial leaflets. EDV-150 ml, ESR-51 ml, EF-57%. Condition after repeated mitral biological valve replacement surgery. Paravalvular 3rd degree regurgitation. 3rd degree tricuspid valve insufficiency (Fig. 1).
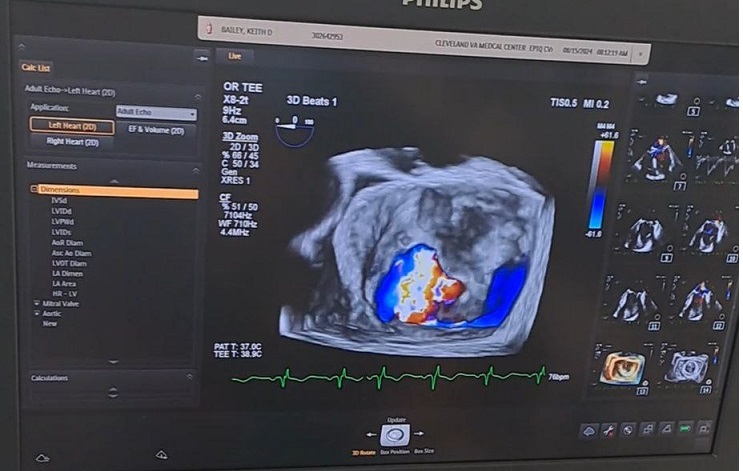 | Figure 1. Paraprosthetic fistula in transesophageal echocardiographic examination |
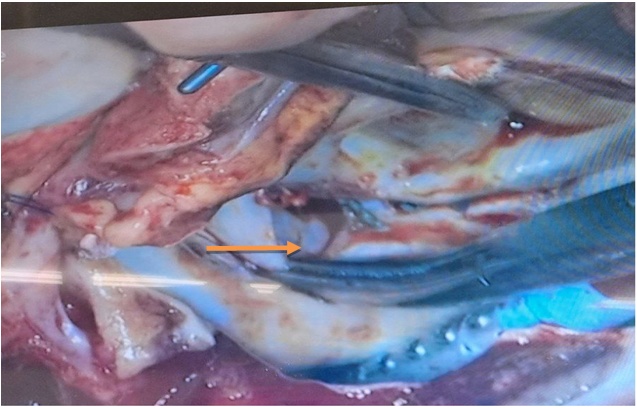 | Figure 2. Intraoperative photographic drawing fistulas mitral valve |
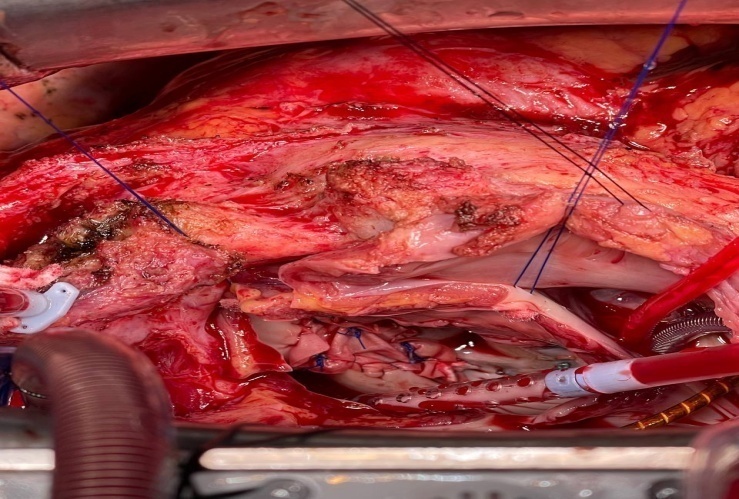 | Figure 3. Closing paraprosthetic fistulas mitral valve using a xenopericardial patch |
3. Conclusions
- Thus, for correction of paraprosthetic fistula there are a number of contraindications, these are such How availability infective endocarditis, valve thrombosis and the risk of prosthesis detachment. When a large paravalvular fistula is detected and calcification occurs around the fibrous ring of the valve after its excision, there is little surrounding material left to perform fistula correction by suturing the edge of the fistula into the cuff of the mechanical valve. For adequate and complete elimination of this situation, it is recommended to use the method “Plastic with a xenopericardial patch”. Using this method, it is possible to avoid intraoperative trauma to dangerous surgical areas, while the application of strong sutures in such clinical situations creates significant technical difficulties. The use of the plastic method with a xenopericardial patch can prevent these technical difficulties and thereby reduce the risk of re-formation of a paraprosthetic fistula. In repeated heart surgeries, MSCT allows one to assess the degree of adhesion formation and a more accurate anatomical location of the fistula, as well as the formation of calcifications around the fibrous ring of the valve. An integrated approach to solving such clinical situations can significantly reduce the risk of developing intraoperative complications using MSCT and transesophageal EchoCG.In general, this case of clinical observation indicates that the diagnosis and surgical treatment of paraprosthetic fistula in repeated operations seems to be quite complex due to the risk of intraoperative complications, as well as the repeated risk of formation of paraprosthetic fistula. In terms of diagnosis of paraprosthetic fistula of the mitral valve and the purpose of improving the quality of technical stages of repeated surgery, it is necessary to use informative and high-tech research methods in the preoperative period (echocardiography, transesophageal EchoCG and MSCT), which make it possible to perform timely and adequate correction of paraprosthetic fistulas. The use of plastic surgery with a xenopericardial patch for paraprosthetic fistula using high-tech diagnostic methods in combination may be a promising method, but further improvement of the surgical technique is required, as well as a study of the remote results of repeated operations.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML