-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 867-871
doi:10.5923/j.ajmms.20251504.03
Received: Feb. 13, 2025; Accepted: Mar. 26, 2025; Published: Apr. 2, 2025

Retrospective Analysis of the Influence of Somatic and Gynecological Diseases on the Development of Hydatidiform Mole
Madrimova Quvonchoy Qahramonovna, Matrizayeva Gulnara Jumaniyazovna, Jumaniyazova Zulhumor Farhodovna
Urgench Branch of the Tashkent Medical Academy, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A very pressing issue in modern medicine is the question of the depth and nature of the similarity between the processes of embryogenesis and carcinogenesis. In recent years, numerous publications have appeared that mainly examine the frequency of trophoblastic tumors and their relationship with pregnancy and childbirth. To date, there is still no clear understanding of the causes of the forms of hydatidiform mole, which significantly complicates the solution of a number of issues related to the diagnosis, treatment and prevention of this pathology. In this regard, further study of various aspects of hydatidiform mole, causes and mechanisms of disease development is necessary.
Keywords: Trophoblastic disease, Hydatidiform mole, Diagnostics, Histological forms of hydatidiform mole
Cite this paper: Madrimova Quvonchoy Qahramonovna, Matrizayeva Gulnara Jumaniyazovna, Jumaniyazova Zulhumor Farhodovna, Retrospective Analysis of the Influence of Somatic and Gynecological Diseases on the Development of Hydatidiform Mole, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 867-871. doi: 10.5923/j.ajmms.20251504.03.
1. The Aim of the Work
- To determine the significance of immunohistochemical research in the diagnosis of malignant transformation of hydatidiform mole in women living in the Aral region, and to develop an algorithm for their management.The results of numerous studies have reliably established the significant role in the genesis of such factors as early first pregnancy, parity of pregnancy, impaired immunity, deficiency of vitamins A and C in food, protein deficiency, inflammatory diseases of the pelvic organs, and genetic predisposition. Timely implementation of preventive measures will reduce the number of relapses and achieve a favorable outcome in subsequent pregnancies [2].Hydatidiform mole occupies a special position in the structure of oncogynecological diseases, as it is directly related to pregnancy. Epidemiological studies have shown the importance of geographic differences in the frequency of this pathology, which varies significantly in ethnic groups of different countries and is interconnected with the socio-economic status and lifestyle of the female population. Among the methods of diagnosing hydatidiform mole, the morphological method is the main one in determining individual forms of the disease [1].As is known, primary manipulations in case of hydatidiform mole (scraping of the uterine mucosa, cesarean section, tubectomy) should be performed during the initial visit of patients to gynecological hospitals and maternity hospitals. Complete hydatidiform mole. The most common symptom is uterine bleeding. Molar tissues can separate from the decidua and destroy maternal vessels, and large volumes of retained blood can expand the uterine cavity. Intrauterine clots oxidize and liquefy, as a result - rusty-colored vaginal discharge that looks like "plum juice". Since uterine bleeding can be large and long-term, half of these patients have anemia, which sometimes requires the use of blood transfusion therapy. Excessive enlargement of the uterus relative to the gestational age is one of the most classic signs of complete hydatidiform mole, although it is found in only half of the patients. The uterine cavity can be expanded both by chorionic tissue and by remaining blood. Excessive growth of the uterus is usually associated with a significant increase in hGG levels [3].Clinically evident hyperthyroidism is found in only 7% of patients, but with a pronounced symptomatic picture, medical care may be required. These patients may have tachycardia, hyperthermia, and tremor. The diagnosis is confirmed by an increase in the level of T3 (triiodothyronine) and T4 (thyroxine) in the blood plasma. Laboratory confirmation of hyperthyroidism is often found in patients with HM in the complete absence of symptoms. If hyperthyroidism is suspected, it is very important to use β-blockers before anesthesia before removal of HM due to the risk of developing a thyrotoxic crisis [4].Thecalutein cysts with a diameter of 6 cm or more develop in approximately half of the patients. They are multi-chamber formations containing amber or serous-hemorrhagic fluid, with bilateral localization. Their formation is associated with an increase in the level of hGG and prolactin [5]. An increase in the ovaries is observed mainly in patients with a significant increase in the level of hGG. Due to the fact that the size of the uterus can also be increased, it may be difficult to palpate thecalutein ovarian cysts, but the use of ultrasound diagnostics can accurately confirm their presence and determine the size. After removal of the HM, thecalutein cysts spontaneously regress within 2-4 months. Thecalutein cysts often cause symptoms of compression of the organs in the pelvis, which can be leveled with laparoscopic decompression or transabdominal aspiration [4].Preeclampsia develops in 27% of cases. Hypertension, proteinuria, and hyperreflexia are often observed in patients, but eclamptic seizures are uncommon. Toxemia occurs mainly in patients with an enlarged uterus and high hGG levels.Partial hydatidiform mole. Partial hydatidiform mole differs significantly from complete hydatidiform mole. Most patients present with signs of a missed abortion or incomplete abortion, and the diagnosis is established only on the basis of tissue examination [5]. Human chorionic gonadotropin is a characteristic secretory product of the trophoblastic cell. Cross-reaction between hGG and luteinizing hormone (LH) is possible due to the fact that they produce indistinguishable α-chains. At the same time, each of the two β-chains is biochemically unique and has biological and immunological specificity. Therefore, one of the most accurate diagnostic methods is radioimmunoassay for the determination of the β-hGG fraction, which is of particular importance in examining patients with gestational trophoblastic neoplasia (HTN), as well as in the quantitative assessment of low hGG levels without the influence of physiological parameters of LH. The diagnosis of complete and partial HM may also include histological examination of the curettage tissue, however, with the diagnosis of "hydatidiform mole", which occurs in the early stages of gestation, doubts may arise. The histopathological characteristics of complete HM in the first trimester are different [3].Immunohistochemical tests can also help confirm the diagnosis of HM, in particular, p57kip2 expression allows differential diagnosis between complete and partial HM. Fluorescent hybridization can be used to differentiate p57kip2-positive partial HM (triploid) from hydropic abortion (diploid). Ultrasonography – a reliable and sensitive method for diagnosing complete hydatidiform mole. Diffusely enlarged and edematous chorionic villi recreate a characteristic picture, which is usually called "Swiss cheese". This method is successfully used to detect complete hydatidiform mole in the first trimester of pregnancy [13]. Ultrasound is used in the diagnosis of partial hydatidiform mole, revealing focal cystic changes in the placental tissues and an increase in the transverse diameter of the ovum [2]. The WHO Scientific Group recommends using the following histopathological definitions (Polyakova V.A., 2001): Hydatidiform mole (HM), this is a general term that includes 2 varieties, namely, complete and partial hydatidiform mole; common morphological features for both forms are edema of individual or all villi and trophoblast hyperplasia. Complete HM. Characterized by the absence of the fetus, marked edema and enlargement of the placental villi with distinct hyperplasia of both trophoblast layers. The edematous villi lead to the formation of a central cistern with simultaneous compression of the maternal connective tissue, which therefore loses vascularization. Partial HM. Characterized by the presence of the fetus, which, however, tends to die early. The placental villi partially swell, which leads to the formation of a cistern and partial trophoblast hyperplasia, usually involving only the syncytiotrophoblast. Intact villi look normal, and the vascularization of the villi disappears following fetal death. Invasive HM is a tumor-like process with myometrium invasion, trophoblast hyperplasia and preservation of the placental structure of the villi. It usually occurs as a result of complete hydatidiform mole, but can also occur with incomplete hydatidiform mole [5]. A simple hydatidiform mole is a grape-shaped formation consisting of transparent vesicles up to 15 mm in diameter and filled with fluid containing albumin and mucin. The vesicles are altered due to edema and mucus formation of the chorionic villi. Usually, all the chorionic villi transform into a hydatidiform mole, which can occupy the entire uterine cavity. The fetus dies in this case. Microscopic examination of a hydatidiform mole reveals proliferation of trophoblast cells and hydropic degeneration of the stroma of the villi. The vesicles of the mole are covered with chorionic epithelium consisting of Langhans cells and syncytium. As the vesicles grow, the chorionic epithelium atrophies, and the sequence of the arrangement of these cell layers is disrupted. Hyperplasia of the chorionic epithelium in the form of a cluster of syncytial cells is noted. There are no blood vessels in the villi. The stroma of the villi is swollen and destroyed collagen fibers. When cut, the vesicle is covered with the remains of the stroma of the villi on the inner surface, and on the outside - with a layer of Langhans cells and a thick layer of syncytial cells with pronounced hyperplasia [2,4]. The degenerated cells of the chorionic villi secrete proteolytic enzymes during disintegration, which melt and thin the decidual membrane at the site of the introduction of the vesicles. In hydatidiform mole, there is no deep invasion and destruction of the myometrium and venous vessels. With pronounced hyperplasia and anaplasia Chorionic epithelium hydatidiform mole often develops into invasive hydatidiform mole. Invasive hydatidiform mole is characterized by intensive proliferation of chorionic epithelium, its anaplasia, and edema of the stroma of the vills. In this form of the disease, syncytiotrophoblast elements deeply grow into the myometrium, destroying it and growing into the venous vessels. The tumor can extend beyond the uterus, spreading into the broad ligament of the uterus, bladder, and abdominal cavity. Histological examination of a scraping from the uterine cavity can only detect hydatidiform mole with intensive proliferation and anaplasia of the epithelium of the chorionic villi. Its relationship to the uterine vessels and myometrium is revealed only when examining a removed macropreparation. When examining a macropreparation, a hydatidiform mole is visible on a cut, growing through the entire or almost entire thickness of the myometrium. Microscopic examination of the tumor reveals smaller villi than in benign hydatidiform mole; chorionic epithelium with pronounced proliferation and anaplasia, syncytial cells are vacuolated; blood vessels are absent or deserted; chorionic villi penetrate deeply into the myometrium and into the wall of the uterine vessels. In the muscular layer of the uterus, foci of hemorrhage and necrosis are found, around which there is lymphocytic infiltration [2,3,5].
2. Material and Method
- This scientific study was conducted from 2022 to 2024 at the Department of Obstetrics, Gynecology and Oncology of the Urgench branch of the Tashkent Medical Academy. Clinical material: • Materials were collected from the Khorezm branch of the Republican Specialized Scientific and Practical Center for Maternal and Child Health and the Republican Specialized Scientific and Practical Medical Center for Oncology and Radiology.• Pathomorphological studies were carried out in the pathomorphology department of this center.• Immunohistochemical studies were carried out in the laboratory of FBC NGS MEDICAL LLC.Data and study groups:1. Retrospective stage of the study:• Uterine aspirate samples from women with hydatidiform mole, collected in 2019–2022, were studied.• Of these, 40 samples underwent immunohistochemical analysis for markers Ki-67, CD34, hCG and p53.2. Prospective stage of the study:• 94 women were included, with an average age ranging from 25.8 to 38.2 years.• Participants lived in the Khorezm region and the Republic of Karakalpakstan (44.6% – urban residents, 55.4% – rural).The women were divided into 3 groups:• Main group (n = 44): women with hydatidiform mole.• Comparison group (n = 30): women with non-viable pregnancies.• Control group (n = 20): practically healthy women who sought a medical abortion.Research methods:• Questionnaire (complaints, medical and life history, menstrual function, reproductive activity, objective examination).• Clinical and laboratory tests: biochemical, hormonal tests, ultrasound results.• Treatment methods and their results.Statistical analysis:To process the data, arithmetic mean values (M), arithmetic mean errors (m) and relative indicators (%) were calculated. Statistical comparisons ensured correct comparison of groups.Tissue sections obtained by curettage or uterine aspiration were made on the same day. The materials were processed using standardized methods of histological examination of biopsy and surgical material using the ThermoFisher histoprocessor. Scientific for 16 hours according to the instructions. Staining of cell nuclei is assessed as follows:• Less than 10% - low activity,• 10-20% - average activity,• More than 20% – high activity.We studied the following immunohistochemical markers: Ki-67, CD34, hCG and p53.
3. Results
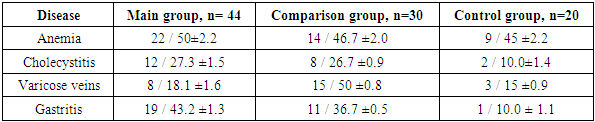
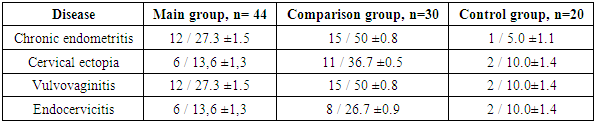
- A study of the somatic disease history of the examined women showed that the most common somatic diseases among all three groups were anemia: in the main group - 50±2.2% (n = 22); in the comparison group - 46.7±2.0% (n = 14); in the control group - 45±2.2% (n = 9).The following were most frequently observed: cholecystitis (main group – 27.3±1.5%, comparison group – 26.7±0.9%, control group – 10±1.4%); gastritis (main group – 43.2±1.3%, comparison group – 36.7±0.5%, control group – 10.0±1.1%). There were also cases of varicose veins (18.1±1.6%, n=8; 15±0.8%, n=15; 15±0.9%, n=3).
|
|
4. Conclusions
- Female patients of reproductive age should remember: in case of any menstrual cycle disorders lasting more than 2 months (amenorrhea, hyperpolymenorrhea, acyclic bleeding) and a history of pregnancy (uterine, ectopic, childbirth, medical and spontaneous abortions), it is always necessary to determine the hGG level in the blood. An elevated hCG level can only be observed during pregnancy or with the development of trophoblastic disease. If pregnancy is not confirmed by ultrasound CT data, it is necessary to urgently consult an oncogynecologist. Histological analysis is the main method for diagnosing hydatidiform mole. Immunohistochemical examination helps to determine the aggressiveness of the disease and choose the optimal tactics for managing patients. Elevated hGG levels are a key diagnostic sign of hydatidiform mole. Women with blood group I (O) have the greatest predisposition to hydatidiform mole. Excessive expression of p53 may indicate a high risk of neoplastic processes requiring further monitoring. The hGG level after evacuation of the hydatidiform mole should be monitored until it completely disappears, since the persistence or re-growth of hGG may indicate residual trophoblastic disease or malignant transformation. A rational approach to examination allows avoiding redundant tests and unnecessary treatment, reducing the burden on the patient.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML