Tashmetova G. T.
Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology, Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
Correspondence to: Tashmetova G. T., Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology, Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan.
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Th1 type inflammation is justified by an increase in the indicators of proinflammatory cytokines IFN-γ and TNF-α and a drop in IL-4 levels at all stages of the disease, which occurred in 29.3% of patients with COPD. Immune mechanisms of the inflammatory response according to the Th1/Th17 phenotype were observed in 26.7% and were characterized by a simultaneous increase in IL-17, IL-21, TNF-α, IFN-γ and IL-6 and a decrease in IL-4 levels. With the aggravation of the COPD stage, the level of TGF-β increases. The formation of an immune response along the Th17–oriented pathway is characterized by a pronounced increase in IL-17, IL-21, IL-6, TGF-β and IL-10, which occurred in 44.0% of patients with COPD. Cytokine levels differed most significantly in patients with extremely severe COPD compared with the control group and stages II-III of COPD.
Keywords:
Chronic obstructive pulmonary disease, Markers of progression, Clinical and immunological phenotypes, Cytokines
Cite this paper: Tashmetova G. T., Clinical and Immunological Phenotypes in the Progression of Chronic Obstructive Pulmonary Disease, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 843-848. doi: 10.5923/j.ajmms.20251503.75.
1. Relevance
According to WHO, vitamin D deficiency is pandemic in nature, covering over 1 billion inhabitants of the planet [1]. Epidemiological statistics on vitamin D deficiency are associated with an increased incidence and risk of diseases mediated by infectious processes, such as acute respiratory infections, pulmonary tuberculosis, inflammatory bowel diseases, and demonstrate a close relationship between low vitamin D levels and adverse outcomes, including indicators of morbidity and mortality, in severe infectious and immuno-mediated diseases [2,3,4,5]. Low vitamin D levels are not the cause of the disease, but a marker or even a consequence of its complicated progressive course [6]. In this regard, the search for the diagnosis of inflammatory markers that lead to the progression of the process is considered relevant.
2. The Aim of the Scientific Research
The aim of the study was to evaluate the systems of regulation of systemic inflammation parameters and immune response in patients with chronic obstructive pulmonary disease in order to clarify the mechanisms of immunoregulatory pathways that determine indicators of disease progression.
3. Research Material and Methods
To achieve our goal, the study included 116 patients with COPD who were undergoing inpatient treatment in the pulmonology department of the Tashkent City Clinical Hospital No. 1, where they underwent comprehensive clinical, laboratory and functional studies within the framework of the set tasks.The type of COPD exacerbation is classified according to Аnthonisen et аl., where type I has 3 major criteria - "the appearance or increase in shortness of breath, an increase in the volume of sputum secreted, an increase in the "purulence" of sputum"; type II - the presence of the first 2 major criteria; type III exacerbation - the presence of 1 major and at least 1 minor criterion, including manifestations of an upper respiratory tract infection, an increase in body temperature above 38°C, increased cough, wheezing [11]. The degree of dyspnea is interpreted according to the discriminatory dyspnea scale MRC (Medicаl Reseаrch Council) [12]. The impact of COPD on the patient's daily life and health was assessed using the COPD Assessment Test (CAT), recommended by GOLD [13], where 0-10 points indicate minor impact; 11-20 points indicate moderate impact; 21-30 points indicate significant impact and 31-40 points indicate severe impact of the disease. The BODE index was used to assess the outcome of the disease, reflecting the percentage of 4-year survival [14].Vitamin D was determined by chemiluminescent immunoassay (CLIA) using a Beckman Coulter kit. The level of cytokines (interleukins (IL)-1; -4; -6; -10; -17; -21; TNF -α; TGF-B) in the serum was determined by the ELISA method on a Mindray Mr-96A spectrophotometer. Specific tissue antibodies to lung tissue were determined by the passive hemagglutination reaction using the generally accepted standardized method.Statistical analysis was performed using the STATISTICA 13.3 program. All values in the tables are presented as the arithmetic mean of the variation series ± the error of the mean (M±m). Values with the level of p<0.05 and p<0.01 (with a confidence probability of 95.5% and 99%) were used as a statistical hypothesis.
4. The Results and Their Discussion
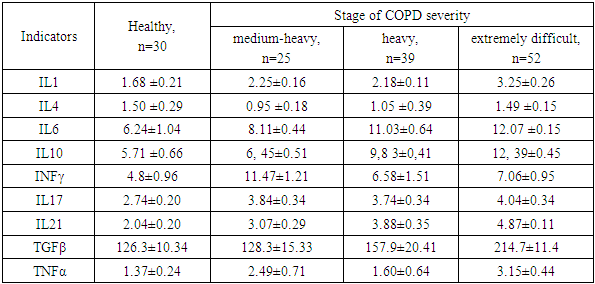
In the course of our own research, we have identified variability in the cytokine profile in patients depending on the severity of COPD (see Table 1). As the stage of the disease worsens, we observe an increase in the levels of cytokines that control inflammation in both T- and B-cell immune responses.Table 1. Cytokine levels in patients with chronic obstructive pulmonary disease
 |
| |
|
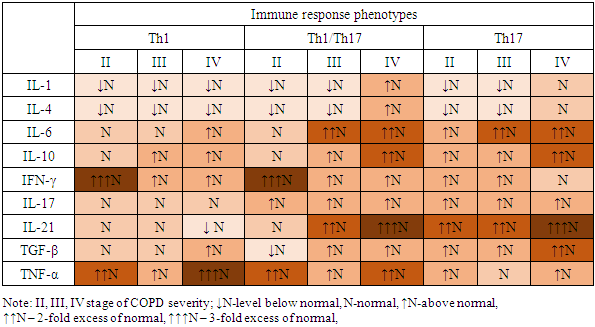
To classify immune response reactions using the cluster analysis method, cytokine levels in COPD patients were differentiated depending on their severity. This allowed us to identify the Th1, Th17, and Th1/Th17 phenotypes, reflecting deviations from the norm of immunological parameters (see cartogram 1).Cartogram 1
 |
| |
|
The results of the study confirm that significant increases in the level of proinflammatory cytokines IFN-γ and TNF-α at all stages of the disease development against the background of IL-4 deficiency predetermine the Th1 pathway of the immune response. In the extremely severe stage of COPD, an increase in IL-6 and TGF-β, specific for the differentiation of Th17 cells, is recorded. Simultaneous significant increase in the levels of pro- and anti-inflammatory cytokines IL-17, IL-21 and TNF-α, IFN-γ enhances the Th1/Th17 immune response and accompanies systemic inflammation at stages II-IV of COPD. In the severe stage of COPD, an increase in IL-6 and a decrease in IL-4 are observed, and in the extremely severe stage, an increase in the level of TGF-β. The most significant deviations in the level of cytokines are found in patients with an extremely severe stage of COPD compared to a cohort of healthy individuals. The Th17 pathway of the immune response is accompanied by an increase in the levels of IL-6, IL-10, IL-17, IL-21 and TGF-β at all stages of the disease, but with the greatest deviation in levels in patients with COPD IV.The frequency of occurrence of different phenotypes of the immune response is associated with the severity of COPD. It was found that among patients with COPD II, the Th1 type of immune response dominates (64% of cases), the Th17-dependent type is less common (24%), and a small proportion of patients have a transitional Th1/Th17 type (12%); in patients with COPD III, Th1, Th1/Th17, and Th17 types of immune response are established with a frequency of 33.3%, 28.2%, and 38.5%, respectively; in patients with COPD IV, the Th17 phenotype is observed in 57.7% of cases, Th1/Th17 in 32.7% of cases, and the Th1 type of immune system response is detected only in 5 patients (9.6%). These data emphasize the relationship between the immune response and the severity of COPD. The Th1 type of immune response dominates in the early stages of COPD II, and the Th17-mediated phenotype is more evident in the later stages of the disease (COPD IV). The Th1/Th17 type is transitional and possibly associated with a more complex clinical course of the disease, and the pattern of cytokine variations highlights the importance of the balance between the phenotypes of the immune response.In patients with Th1 type of immune response, shifts in cytokine levels and profile were determined not only relative to the normal values of the control group (p<0.05), but also by the severity of COPD and were accompanied by variations in the increase or decrease of some cytokine parameters, where in patients with COPD II-IV, an increase in IFN-γ levels was noted by 220%, 52% and 92%, respectively; an increase in TNF-α by 100%, 31% and 225%, respectively, and a decrease in IL-4 levels by 42% in COPD II and a decrease in the indicator by 28% from the norm in COPD III. It was noted that in patients with COPD II I, an increase in IL-10 levels by 93%. In COPD IV, an increase in IL-6 levels by 66%, TGFβ1 by 42%, and a decrease in IL-21 levels by 34% were observed compared to the control group (Fig. 1).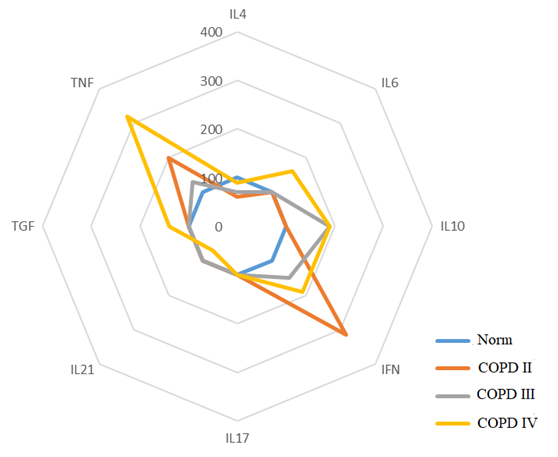 | Figure 1. Th1 Phenotype of the Immune Response |
In patients with Th1/Th17-oriented immune response in COPD, there is a change in the level of cytokines compared to the control group. In particular, in COPD II, there is an increase in IFN-γ by 215%, TNF-α by 117% and IL-17- by 64%, as well as a decrease in the level of IL-4 by 38%. In COPD III, there is an increase in the levels of cytokines IL-21 by 164%, IL-6 by 114%, IL-17 by 76% and IFN-γ- by 55%, and a decrease in the level of IL-4 by 31%. In the case of COPD IV, an increase in the level of IL-21 indicators by 206%, IL-6 by 126%, TGF-β1 by 73%, IL-17 by 66%, TNF-α by 114%, IFN-γ by 42% and IL-10 by 120% of the control norm is detected (Fig. 2).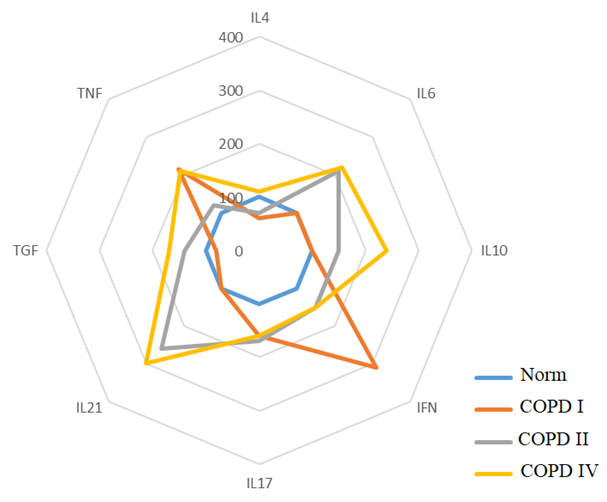 | Figure 2. Th1/Th17 Phenotype of the Immune Response |
With the Th17 phenotype of the immune response, changes in cytokine levels were noted both relative to the control group (p < 0.05) and the severity of the disease, where in patients with COPD II it is associated with an increase in IL-21 levels by 15.4%, IL-6 by 9.1%, IL-17 by 68%, IL-10 by 44% and TNF-α by 3.6% with a simultaneous decrease in IL-4 levels by 33%. With COPD III, an increase in IL-21 levels by 177%, IL-6 by 129%, IL-10 by 80%, IL-17 by 41%, TGF-β1 by 37% is observed, as well as an increase in IL-4 to the control level. Patients with COPD IV are characterized by a more pronounced increase in the level of indicators: IL-21 by 251%, IL-10 by 125%, TGF-β by 107%, IL-6 by 103%, IL-17 by 82% and TNF-α by 63%. The identified changes indicate differences in the immune response between the stages of the disease. (Fig. 3).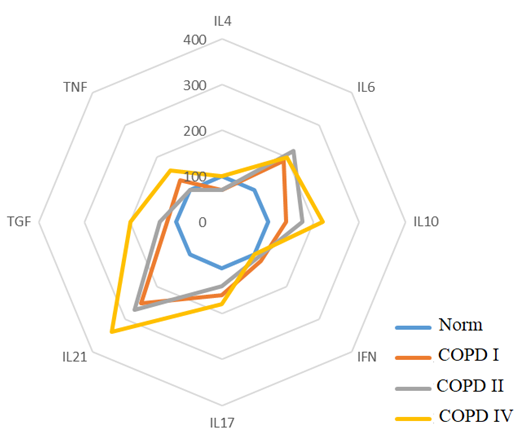 | Figure 3. Th17 Phenotype of the Immune Response |
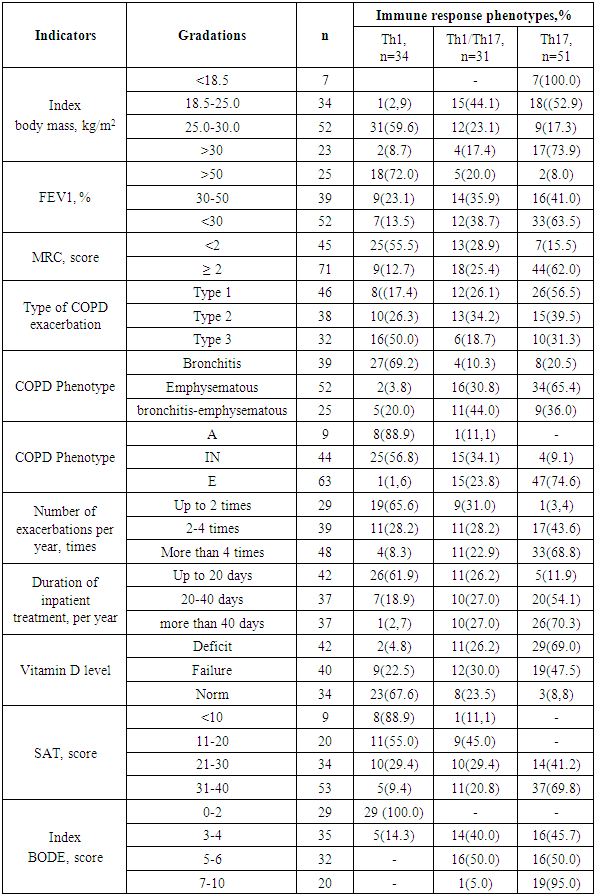
Clinical and functional indicators identifying the progressive course, outcome of COPD and its impact on the patient's daily life and health, including the severity of obstruction, the severity of dyspnea, the phenotype of the disease and the status of vitamin D deficiency and sarcopenia were compared with the characteristics of the immunoregulatory pathways of systemic inflammation (Table 2).Table 2. Clinical and functional indicators of COPD in patients with different immune response phenotypes
 |
| |
|
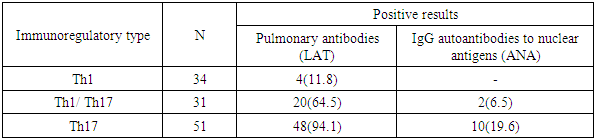
Clinical and functional indicators of COPD in patients with a Th1-oriented type of immune system response in the overwhelming majority of cases were determined by the degree of dyspnea up to 2 points and moderate obstructive disorders, bronchitis phenotype and exacerbation type according to Antonisen III, frequency of exacerbations no more than 2 times a year and duration of inpatient treatment no more than 20 days per year, insignificant level of the indicator of the impact of COPD on the patient's daily life and health and high 80 percent of 4-year survival. In patients with COPD with a Th1-oriented type of immune response, normal levels of vitamin D in the blood serum were noted.In the vast majority of patients with the Th17 phenotype of the immune response, the course of COPD was characterized by a high degree of dyspnea at rest and with light physical exertion (62.0%), severe (41.0%) and extremely severe (63.0%) obstructive disorders, emphysematous phenotype (65.4%) with type I exacerbations (56.5%), a frequency of exacerbations of more than four times a year (68.8%), a significant and severe indicator of the impact of the disease on the daily life and health of patients, a high rate of only 4-year survival (18%), as well as concomitant vitamin D deficiency and sarcopenia, characterized by depletion of muscle tone and weight loss.Evaluation of the immunoregulatory mechanism of inflammation in COPD allows identifying defects when the immune system is involved and triggers an autoimmune process of response to its proteins as foreign. The results of the study showed that 62.1% (72 patients) of COPD have an increase in pulmonary antibody titers in the range of diagnostic levels from 1:44 to 1:320 U.S. units, and 10.3% (12 patients) have positive results for IgG autoantibodies to nuclear antigens (ANA Hep Screen), the degree of expression of changes in which is more often observed in patients with the Th17 phenotype of the immune system response (Table 3).Table 3. Assessment of the immunoregulatory mechanism depending on positive titers of pulmonary antibodies and IgG autoantibodies to nuclear antigens
 |
| |
|
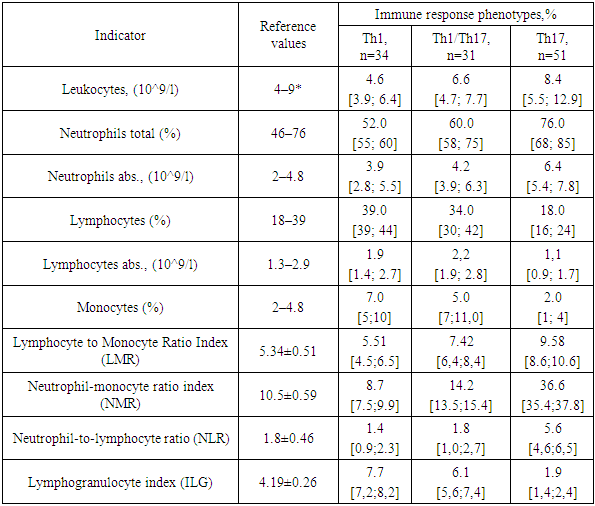
The ANA test is considered a very useful method for identifying autoimmune conditions even in the early stages of the disease, which is important for determining the treatment of the condition and predicting the outcome of the disease.A comparative analysis of hematological leukocyte index data with immunoregulatory types of systemic inflammation in patients with COPD is presented in Table 4.Table 4. Comparative analysis of leukocyte formula indicators, gematological leukocyte indices in patients with COPD
 |
| |
|
The study established that Th1/Th17 and Th17-oriented types of immune response are characterized by the predominance of the activity of the microphage system and the intensity of the effector link of the immunological process, manifested by the suppression of the indicators of the cellular link of immunity and the activation of the humoral link and correlate with an increase in the main hematological leukocyte indices ISLM by 1.6 times, ISLM by 4.4 times, ISNL by 3.3 times compared to the indices of the cohort of COPD patients with a Th1-mediated immune response.
5. Conclusions
Thus, high tension of non-specific link of immunity stimulates development of autoimmune nature of disorders, which is characterized by significant decrease of IPH by more than 3.3 times with Th17 phenotype of immune response. Comparison of hematological leukocyte indices with cytokine profile revealed presence of inverse correlation of IPH with IL-4 (r=-0.55) and IL-10 (r=-0.51); ISNM with TNFα (r=-0.47).
References
| [1] | Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017 Jun; 18(2): 153-165. doi: 10.1007/s11154-017-9424-1. |
| [2] | Ibrayeva L.K., Rybalkina D.Kh., Evmenova O.A., Turdaly F.M., Ibragim Zh.T. D-hypovitaminosis in working age patients with diseases of the respiratory organs: the risks and prevention. Pul’monologiya. 2022; 32 (6): 891–898 (in Russian). DOI: 10.18093/0869-0189-2022- 32-6-891-898. |
| [3] | Velikaya O.V., Vasilieva L.V., Nedomolkina S.А., Nedomolkin S.V. Correlation between bronchopulmonary diseases and vitamin D level. Tuberculosis and Lung Diseases, 2020, Vol. 98, no. 11, P. 57-64. (In Russ.) http://doi.org/10.21292/2075-1230-2020-98-11-57-64. |
| [4] | Dolgo-Saburova YuV, Zazerskaya IE, Dorofeykov VV. Vitamin D and anti-infectious immunity. Choice of diagnostic and treatment monitoring method of vitamin D deficiency. Laboratory Service. 2021; 10(2): 4754. (In Russ.), https://doi.org/10.17116/labs20211002147. |
| [5] | Aibana O. et al. Vitamin D status and risk of incident tuberculosis disease: A nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med. 2019 Sep 11; 16(9): e1002907. doi: 10.1371/journal.pmed.1002907. eCollection 2019. |
| [6] | Bergman P. Low vitamin D is a marker for poor health and increased risk for disease: But causality is still unclear in most cases. J Intern Med. 2023 Mar; 293(3): 272-274. doi: 10.1111/joim.13582. Epub 2022. |
| [7] | Atyakshin D.A., Tsvetikova L.N., Lobeeva N.V., Budnevsky A.V., Ovsyannikov E.S. Indicators of immune status in chronic obstructive pulmonary disease // Advances in modern natural science. – 2015. – No. 9-2. – pp. 195-197. |
| [8] | Cypowyj S., Picаrd C., Mаródi L., Cаsаnovа J.-L., Puel, А. Immunity to infection in IL-17-deficient mice аnd humаns // Eur. J. Immunol., 2012. - 42, 2246–2254. |
| [9] | Kosyakova N.I. et al. Clinical and immunological features and enzymes of cell energy metabolism in COPD with frequent exacerbations // International Journal of Applied and Basic Research No. 8, 2022. - pp. 25-31. |
| [10] | Novikov D.K. Immunological phenotypes of chronic obstructive pulmonary disease: prospects for immunocorrection // Clinical Immunology, Allergology. - 2014. - P. 102-104. |
| [11] | Anthonisen NR, Connett JE, Enright PL, Manfreda J; Lung Health Study Research Group. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002 Aug 1; 166(3): 333-9. doi: 10.1164/rccm.2110093. PMID: 12153966. |
| [12] | Sunjaya A, Poulos L, Reddel H, Jenkins C. Qualitative validation of the modified Medical Research Council (mMRC) dyspnoea scale as a patient-reported measure of breathlessness severity. Respiratory Medicine. 2022 Nov 1; 203: 106984. |
| [13] | Mаhler D.А. et аl. Measurement of dyspnea and quality of life in advanced lung disease. Clinics in Chest Medicine. Volume 18, Issue 3, 1997, Pages 457-469. |
| [14] | Formiga MF, Vital I, Urdaneta G, Balestrini K, Cahalin LP, Campos MA. The BODE index and inspiratory muscle performance in COPD: Clinical findings and implications. SAGE Open Med. 2018 Dec 12; 6: 2050312118819015. doi: 10.1177/2050312118819015. PMID: 30574307; PMCID: PMC6295678. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



