-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(3): 840-842
doi:10.5923/j.ajmms.20251503.74
Received: Mar. 3, 2025; Accepted: Mar. 26, 2025; Published: Mar. 31, 2025

Analysis of the Association of the Glu429Ala Polymorphism of the MTHFR Gene in the Development of Chronic Heart Failure in Patients with Coronary Heart Disease
Musashaykhov U. Kh.1, Makhsudov O. M.1, Aripov O. A.2, Boboyev K. T.3
1Andijan State Medical Institute, Uzbekistan
2Center for Professional Qualification Development of Medical Workers, Uzbekistan
3Republican Specialized Scientific and Practical Medical Center of Hematology, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study evaluated the role and significance of the Glu429Ala polymorphic markers in the MTHFR gene in the development of chronic heart failure (CHF) in 103 patients with ischemic heart disease (IHD). The actual distribution of genotypes of the Glu429Ala polymorphism of the MTHFR gene in the studied groups corresponded to the expected indicators according to the Hardy-Weinberg equation (HWE) (p<0.05). When studying the effect of alleles and genotypes of the Glu429Ala polymorphism of the MTHFR gene in patients with II-IV FС of CHF. It was found that carriers of the Ser allele and Pro/Ser heterozygous genotype have an increased risk of developing CHF.
Keywords: Chronic heart failure, IHD, Genotypes , Glu429Ala polymorphism in the MTHFR
Cite this paper: Musashaykhov U. Kh., Makhsudov O. M., Aripov O. A., Boboyev K. T., Analysis of the Association of the Glu429Ala Polymorphism of the MTHFR Gene in the Development of Chronic Heart Failure in Patients with Coronary Heart Disease, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 840-842. doi: 10.5923/j.ajmms.20251503.74.
Article Outline
1. Introduction
- Chronic heart failure (CHF) is a syndrome that develops as a result of impaired myocardial ability to fill and/or empty, which in turn is accompanied by inadequate perfusion of organs, systems, and tissues. This manifests in symptoms such as fatigue, weakness, shortness of breath, and, as the condition progresses, edematous syndrome. CHF, being the outcome of all cardiovascular diseases (CVD), represents one of the significant challenges in modern healthcare. According to epidemiological data, the global prevalence of CHF is 2.4%, with its frequency increasing with age (among individuals over 45 years, it reaches 2.5%), and approximately 50% of patients, despite the use of combination therapy, die within 5 years [3].The prevalence of chronic heart failure (CHF) continues to steadily increase, as CHF represents the outcome of the so-called cardiovascular continuum and remains one of the primary challenges in clinical cardiology. Over the past decade, CHF has garnered heightened attention from cardiologists. This is attributed to five main reasons: the growing number of patients with CHF, the poor prognosis of the disease, the rising number of hospitalizations due to CHF exacerbations, the unsatisfactory quality of treatment, and the increasing costs associated with managing CHF [2].CHF is closely linked to disturbances in myocardial metabolic processes, intracardiac and peripheral hemodynamic changes, and structural remodeling of the heart. Clinical diagnosis, disease stages and progression, as well as survival rates, life expectancy, and the risk of developing various cardiovascular complications in patients, are increasingly regarded as the most critical criteria for assessing the significance of specific factors in CHF [1].It is becoming clear that there is a need to search for effective methods of early diagnosis and successful treatment of cardiac decompensation. Studies in recent years have shown that 16% of patients with chronic heart failure (CHF) experience an exacerbation of decompensation within the first month after discharge from the hospital, while 37% experience it within the first three months of follow-up [4].It is becoming clear that there is a need to search for effective methods of early diagnosis and successful treatment of cardiac decompensation. Studies in recent years have shown that 16% of patients with chronic heart failure (CHF) experience an exacerbation of decompensation within the first month after discharge from the hospital, while 37% experience it within the first three months of follow-up [5,6].These data highlight the significant importance of studies aimed at elucidating the mechanisms of the formation and progression of chronic heart failure (CHF), contributing to the development of new approaches for timely preventive measures against cardiovascular diseases through the early and accurate diagnosis of risks associated with pathological myocardial changes. This confirms the relevance of this topic.
2. Main Body
2.1. Purpose of the Study
- To study the frequency of distribution and evaluate the relationship of the Glu429Ala polymorphism in the MTHFR gene in patients with chronic heart failure.
2.2. Material and Methods of Research
- For our study, 103 patients with CHF were recruited. These patients were hospitalized in the 1st therapy department of the clinic of the Andijan State Medical Institute. Clinical and laboratory examinations were performed at the Republican Specialized Scientific and Practical Hematology Center under the Ministry of Health of the Republic of Uzbekistan.The diagnosis of CHF was made based on current clinical recommendations. For this, clinical and laboratory examinations were performed, including: anamnesis data, patient complaints and clinical examination results, 6-minute walk test, laboratory tests - biochemical blood analysis, High-tech laboratory studies: molecular genetic analysis. Genotyping of polymorphism Glu429Ala polymorphism in the MTHFR It was carried out on the basis of the method of Tag Man probes on an amplifier Rotor-Gene Q (Quagen Germany), using the commercial test kit of Syntol LLC (Russia).Statistical processing of the results was performed using a standard application software package OpenEpi V.9.2. Analysis of the deviation of empirical genotype frequencies from The theoretically expected Hardy-Weinberg distribution was performed using the Statistica 6.0 software package.
2.3. The Results Obtained and Their Discussion
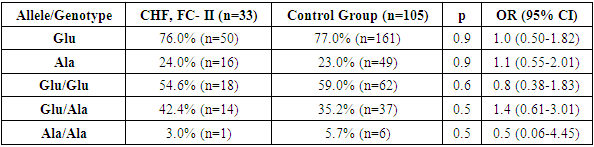
- Comparative analysis of the allele frequency distribution of the MTHFR gene Glu429Ala polymorphism in samples from patients with CHF, FC II, and comparison groups showed that in this group of patients, the proportion of the wild-type Glu allele was 76.0%, which was insignificantly lower than in the control group, while the mutant Ala allele was detected in 24% of cases and was insignificantly higher than in the reference group. In the control group, these alleles were 77.0% and 23.0%, respectively (χ² = 0.02; p = 0.9; OR = 1.0; 95% CI: 0.50–1.82 and χ² = 0.02; p = 0.9; OR = 1.1; 95% CI: 0.55–2.01) (Table 1).
|
|
|
3. Conclusions
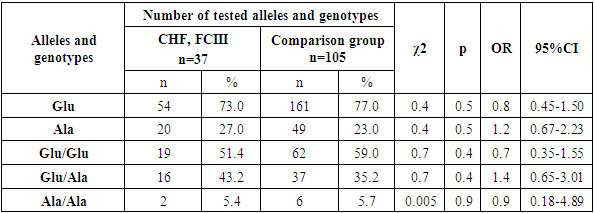
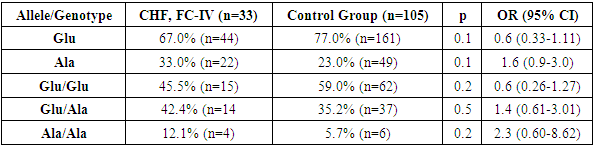
- Thus, in the group of patients with CHF and FC IV, an increase in the proportion of the minor Ala allele and the unfavorable Ala/Ala genotype was observed compared to the CHF FC II-III group and the group of conditionally healthy donors. This, in turn, indicates that in patients with CHD, the detection of the minor Ala allele and the unfavorable Ala/Ala genotype of the MTHFR gene Glu429Ala polymorphism is associated with the likelihood of CHF development and a more severe clinical course.The results of these analyses further confirm the importance of genetic testing in developing individualized approaches for conditions associated with CHD and CHF.Such scientific investigations contribute to a better understanding of the pathogenesis of diseases and the development of effective treatment methods.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML