-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(3): 711-716
doi:10.5923/j.ajmms.20251503.49
Received: Feb. 5, 2025; Accepted: Mar. 2, 2025; Published: Mar. 15, 2025

The Role of the Complement System in Herpetic Stromal Keratitis Caused by the Herpes Simplex Virus Type 1 in Children
M. M. Tabibova, A. A. Khadjimetov, B. T. Buzrukov, U. T. Nugmanova
Tashkent State Dental Institute, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of this study was to determine the presence and functional activity of the complement cascade in tears in inflammation caused by the herpes simplex virus. The object of the study was 48 tear fluid samples taken from 48 children (25 girls, 23 boys) aged 10 to 17 years (average age 13.1±2.2 years). The control group consisted of lacrimal fluid samples from 9 children without eye pathology. In lacrimal fluid samples, the content of complement components (C3, C4, and C5) was determined in lacrimal fluid samples obtained from healthy people and from patients with inflammation caused by the herpes simplex virus by the immune enzyme method. In children with herpetic stromal keratitis caused by the herpes simplex virus, changes in the concentration of complement components C3, C4 and C5a in the lacrimal fluid were noted, which can cause frequent intraoral infection of the herpes simplex virus and activate immune cells in the corneal epithelial cells with the release of anaphylatoxins C3a and C5a.
Keywords: HSV-1, Herpetic keratitis in children-complement components- C3, C4, C5
Cite this paper: M. M. Tabibova, A. A. Khadjimetov, B. T. Buzrukov, U. T. Nugmanova, The Role of the Complement System in Herpetic Stromal Keratitis Caused by the Herpes Simplex Virus Type 1 in Children, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 711-716. doi: 10.5923/j.ajmms.20251503.49.
Article Outline
1. Introduction
- Childhood blindness is a life-changing condition for children all over the world. To solve the problem of the estimated prevalence of 1.4 million irreversibly blind children at that time, the World Health Organization considered the control of blindness in children to be one of the main priorities of its VISION 2020 — The Right to Vision campaign, which was launched in 1999 [39]. More recent estimates of prevalence as of 2020 range from 1.02 to 1.44 million, depending on methodology, with 22.16 million people estimated to have moderate to severe visual impairment and 46.60 million to have mild visual impairment [5]. Maintaining vision is important because it plays a crucial role in infancy and childhood, during the formative years when children learn to synthesize sensory information and interact with the world. Vision deficiency can disrupt early development and learning, leading to lifelong intellectual, emotional, and social consequences [43,3].Herpes simplex virus type 1 (HSV-1) is a common human pathogen that has affinity for the mucosal epithelium as the first line of replication before spreading proximally to other cell types and distally to the peripheral and central nervous system. Herpetic stromal keratitis is a chronic inflammatory disease involving innate and adaptive immune responses. Although the induction of reliable innate interferon (IFN) responses is crucial for limiting HSV-1 replication in the corneal epithelium, these IFN-mediated responses also activate adaptive immune branches, contributing to inflammation and vision loss.The innate immune system, consisting of cells of myeloid origin and soluble antiviral factors, is recruited and produced accordingly in response to the initial HSV-1 lesion. Chemokines are also key molecules that play an indirect role in the pathogenesis and recruitment of leukocytes in the early stages after infection of the cornea with HSV-1 [36,11,26]. HSV-1 is known to encode immune response evasion proteins, including glycoprotein C (gC), which binds to the C3 protein, preventing complement-mediated lysis of virus-infected cells and blocking complement-mediated virus neutralization [32,38,4]. Antigen-specific IgG is an important protection against HSV-1, and complement contributes to its effectiveness in neutralizing the virus [34]. It is still unknown whether HSV-1 regulates the metabolism of immune cells through the expression of its viral proteins in order to evade antiviral immunity.The main component of innate human immunity is the complement system. The most studied function of this humoral system, which consists of a cascade of proteases and soluble factors, is innate immunity and the destruction of microbes. However, for more than a decade, the complement system has been involved in a variety of processes during development, degeneration, and regeneration [12-15,1,37,31,44]. The complement cascade can be activated in three different ways: classical, alternative, and lectin. The role of complement in metabolism and metabolic disorders has recently come to the fore and has received increasing scientific attention. The initial stages of the development of the complement system involve the activation of proenzymes in a cascade reaction, while the assembly of the membrane attacking complex leads to cell lysis. The presence of complement components in tears has been the subject of some controversy. Studies have shown the presence of individual components 5,6, but the presence of a common pathway has not been shown. 7.8 components of complement and regulatory proteins were detected in the corneal tissue, while the composition of the tear film changes during sleep. Open eyes and reflex tears consist mainly of lysozyme, lactoferrin, lipocalin (prealbumin), and slgA. During sleep, a constituent tear fluid is produced, consisting of slgA (up to 80% of the total protein) and elevated levels of proteins derived from blood serum 9. It should be noted that C3 is activated during sleep. One of the proteins obtained from blood serum is vitronectin, 10 which can weaken the activation of complement in tears. Complement plays a well-known role in maintaining a healthy cornea [6]. This is important because the surface of the cornea is constantly exposed to various pathogens, including bacteria such as Pseudomonas aeruginosa [4], and, consequently, the continuous activation of complement. The present study was undertaken to expand on these results by evaluating the immediate host response to HSV-1 corneal infection, with a focus on the complement system and the IFN type 1 response, since these two pathways represent ancient innate defense mechanisms [39,2,16] and at the same time, IFN type 1, including IFN-α and -β, are powerful antiviral cytokines that block HSV-1 replication [23,8]. Therefore, it follows from the above that detailed studies are currently needed to understand the direct and indirect role of viral proteins in evading the antiviral immunity of the host. Moreover, there is an urgent need to identify clinical (diagnostic and translational) biomarkers and highly sensitive rapid screening assays/tests that can differentially diagnose eye infections with various pathogens. The purpose of this study was to determine the presence and functional activity of the complement cascade in tears in inflammation caused by the herpes simplex virus.
2. Research Materials and Methods
- The object of the study was 48 tear fluid samples taken from 48 children (25 girls, 23 boys) aged 10 to 17 years (average age 13.1±2.2 years). The control group consisted of lacrimal fluid samples from 9 children without eye pathology. Advanced diagnostic methods included polymerase chain reaction (PCR) - traditional PCR, reverse transcriptase PCR (RT-PCR), real-time PCR (KPCR) and multiplex PCR performed on corneal scrapings. The sensitivity and specificity of traditional PCR were 100% and 76.9% and 100% and 28.2% PCR, respectively. LV was collected in the morning hours after irritation of the nasal mucosa by inhalation of a 10% ammonium hydroxide solution from the lower conjunctival arch of one eye with a microcapillary in a volume of 100 µl and placed in a dry sealed tube. Immediately after sampling, the fat was frozen at a temperature of - 18°C. Patients with inflammation caused by the herpes simplex virus did not receive any eye treatment, and they were told not to instill eye drops on the day of tear collection. In lacrimal fluid samples, the content of complement components (C3, C4, and C5) was determined in lacrimal fluid samples obtained from healthy people and patients with inflammation caused by the herpes simplex virus using the immune enzyme method using HUMAN reagents on a MINDRAY analyzer (China).For cytokine analysis, tear samples were diluted in ELISA buffer (supplied by the manufacturer) to a final volume of 100-200 µl. These analyses were carried out in accordance with the manufacturer's recommendations. The data was expressed as an average value ± the standard error of the mean (SEM). The statistical analysis of the obtained data was carried out using the Microsoft Excel program and the STATISTICA 6.0 statistical software package. The significance of differences in the average values of quantitative features was assessed by nonparametric methods using the Mann-Whitney U—test for two independent groups. The differences were considered significant in the case of p < 0.05.
3. Research Results
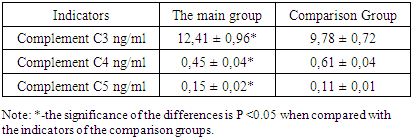
- Human herpes simplex virus-1 (HSV-1) is a highly effective neurotropic pathogen that infects the epithelial cells lining the mucous membrane of the cornea of the eye. After primary lytic replication in epithelial cells of the corneal mucosa, HSV-1 establishes lifelong latency in neurons in the trigeminal ganglion. Children with weakened immune systems often experience reactivation of HSV-1 from latency, which leads to the virus entering sensory neurons, followed by anterograde transport and lytic replication on the innervated surface of the mucosal epithelium. Although recurrent infection of the surface of the corneal mucosa is rare, it can lead to a chronic immune-inflammatory condition called herpetic stromal keratitis (HSK). HSK leads to gradual loss of vision and can cause permanent blindness in severe untreated cases.The activation of the complement system (CS) occurs within a few seconds after damage by the pathogen, enhancing phagocytosis of complement-loaded pathogen-associated antigens or pathogens (for example, virus), contributing to the neutralization of antibodies, facilitating disruption of the integrity of the pathogen through the development of a membrane attack complex and helping to attract macrophages and neutrophils [20]. Thus, the results suggest a protective role of C3, delaying corneal and NV inflammation after HSV-1 infection. CS is crucial for maintaining and balancing immune homeostasis through its strong association with innate protection against infection by a microbial pathogen [14,33]. The above highlights the regulatory role of complement in corneal health and diseases [17]. As can be seen from the presented results of the study (Table 1), the content of the complement component C3 in the lacrimal fluid in herpetic stromal keratitis caused by the herpes simplex virus in children is increased by 30% relative to the indicators of the comparison groups. As is known, the central component of the complement system is the complement component C3. C3 is a critically important component of the innate and adaptive immune system, which directly contributes to the protection of the patient through the enzymatic generation of C3b, which leads to the formation of C5 convertase and C5b to launch the terminal pathway, the membrane attack complex [43]. In turn, C3b leads to opsonization [3] and activation of T cells through the intracellular pathways of the complement system [36,11]. C3 also plays a role in virus purification by coating the virion, promoting proteasome degradation and thus preventing virus replication [3]. It should be noted that against the background of an increase in the level of the complement component C3 in the lacrimal fluid, as shown in Table 1, the replication of the herpes simplex virus decreases [38], i.e. the intact complement system suppresses corneal inflammation by the herpes simplex virus-1 by activating phagocytosis. The slight increase in the content of the complementa C3 component that we observed does not seem to be related to the viral antigen per se, but rather reflects the effect of the complement system on inflammation in response to a local viral infection [12]. According to some researchers, a decrease in the level of complement C3 can lead to increased neovascularization, corneal opacity and increased inflammation, as well as an increase in inflammatory immune cells and pro-angiogenic and pro-inflammatory cytokines infected with HSV-1 [41].
|
4. Discussion
- All herpes viruses are capable of reaching a state of latency where the virus remains inactive in cells and reactivates from time to time. Recurrence can be described as the most characteristic feature of HSV-related corneal infections, subsequently leading to blurred vision and blindness. According to epidemiological data, HSV-keratitis remains the leading infectious cause of blindness in the world. The global incidence of HSV keratitis is estimated to be approximately 1.5 million, including 40,000 new cases each year. In addition, the recurrence rate is high. It was estimated to be 9.6% after 1 year, 22.9% after 2 years, and 63.2% 20 years after the first episode of documented keratitis caused by the herpes simplex virus. [34,20,9,7]. In addition, the level of seroprevalence is high worldwide and is estimated to be above 50%. The general pathogenesis of herpesvirus infections includes: active virus replication, latency status, and reactivation. The primary infection, usually in childhood, can be asymptomatic, oral, but it can also affect the upper respiratory tract or the surface of the eye in the form of conjunctivitis or blepharoconjunctivitis.HSV-1 encodes a number of immune evasion molecules that disrupt the processes of the innate and adaptive immune system [30,27]. From the complement point of view, HSV-1 glycoprotein C binds to human complement component 3 (C3) and prevents complement-mediated virus neutralization and lysis of infected cells [18,35]. It was found that inside the cornea, C3 promotes denervation mediated by HSV-1 [21]. Activation of the complement system (CS) neutralizes viruses through; viral opsonization by complement components; virolysis, which occurs when the membrane attack complex (MAC) produces holes on the viral membrane; and anaphylatoxin production [29,14,17]. Virus neutralization occurs due to the deposition of complement proteins on viral surfaces, which can block the interaction of the virus with the host receptor. This can additionally cause aggregation of viral particles and induce an antiviral state, as well as enhance phagocytosis [15,10,27,13]. The virus can then enter a latent state inside the trigeminal ganglion and remain there throughout the host's life. Under the influence of various stimuli, the virus can reactivate from its latent state and produce live infectious virions, which are delivered and cause immune reactions in the distal areas (cornea) [35,21]. This process leads to a lifelong battle between HSV-specific CD8+T cells and neurons containing HSV. When is the CD8+ functionalityT cells are disrupted due to immune suppression, HSV-1 can break out of its latent state, return to the cornea and cause disease [28,29,2]. This process is progressing, and it takes years to develop after numerous rounds of viral reactivation from latency. The normal cornea expresses various complement components, including C1, C2, C4, C5, C6, C7, properdin, and factor B, which further confirms the importance of the complement system in maintaining corneal health [28]. In addition, the corneal layers express several complement regulatory proteins, such as DAF and CD59, which prevent excessive complement activation and subsequent tissue damage [18,40]. On the contrary, C3 deficiency leads to increased neovascularization, corneal opacity and increased inflammation, as well as an increase in inflammatory immune cells and pro-angiogenic and pro-inflammatory cytokines. It should be noted that the complement system in the body is tightly regulated by the expression of complement regulatory proteins, which prevent complement activation under normal conditions in order to preserve the architecture of the eye surface and maintain visual acuity [1,37]. Hematopoietic cells, including macrophages and type 1 interferons, also cause resistance to the herpes simplex virus. As is known, the normal cornea expresses various components of complement, including C1, C2, C4, C5, C6, C7, properdin, and factor B, which further confirms the importance of the complement system in maintaining corneal health [27]. In addition, the corneal layers express several complement regulatory proteins, such as DAF and CD59, which prevent excessive complement activation and subsequent tissue damage [19]. However, in some diseases of the cornea, endogenous enzymes can destroy these regulatory complement proteins, leading to excessive inflammation and tissue damage [14]. While the inflammatory response mediated by the innate immune system can be devastating and lead to neurological consequences, the host's immune response is crucial for controlling and defending against the virus. Both the innate and adaptive immune systems determine the severity of the disease, the degree of damage, latency, and recurrence. It has been shown that recurrent infections, corneal scarring, and mortality are more significant in immunocompromised patients [24]. Recent clinical studies have revealed that patients with IRF3 genetic defects show increased susceptibility to viral infection in the central nervous system due to reduced IFN production when TLR3 is stimulated by HSV-1 [26]. The secretion of antiviral cytokines as a result of immune activity is crucial for the elimination and control of the virus, although the inflammatory reaction it causes can lead to harmless pathologies. Complement turnover in the eye is essentially about the balance between controlled, slow, ticking, which promotes immune tolerance and stability, without losing the ability to respond when necessary to protect host tissues and remove unwanted immune complexes. Any violation of this delicate balance can lead to dysregulation, which contributes to inflammatory conditions of the eye. Regarding the exciting work currently underway in the field of AMD and new therapeutics, it is important that instead of diving headlong into new therapeutic strategies, we take the time to review the unique anatomy of the human eye and carefully consider where to eliminate excessive complement activation and at what stage of disease progression therapy should be performed.
5. Conclusions
- 1. In children with herpetic stromal keratitis caused by the herpes simplex virus, an increase in the content of the complement component C3 in the lacrimal fluid was noted by 30% relative to the indicators of the comparison groups.2. Indicators of C4 in the lacrimal fluid in children with herpetic stromal keratitis caused by the herpes simplex virus were reduced by 26% relative to baseline values, which can cause frequent intraoral infection of the herpes simplex virus.3. Complement activation leads to the attraction and activation of immune cells mediated by complement activation in corneal epithelial cells and the release of anaphylatoxins C3a and C5a.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML