-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(3): 587-590
doi:10.5923/j.ajmms.20251503.21
Received: Feb. 3, 2025; Accepted: Feb. 23, 2025; Published: Mar. 8, 2025

Method of Improving the Training Treatment Program in Polycystic Ovary Syndrome
Kh. B. Sattaraliyeva
Andijan State Medical Institute, Fergana Institute of Public Health, Uzbekistan
Correspondence to: Kh. B. Sattaraliyeva, Andijan State Medical Institute, Fergana Institute of Public Health, Uzbekistan.
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the recent past, the term “polycystic ovary syndrome” (PCOS) was understood as a pathological condition of the reproductive system, characterized by a certain change in the structure and function of the ovaries, which is based on a violation of the hypothalamic regulation of the secretion of gonadotropic hormones. Polycystic ovary syndrome is one of the main causes of infertility and is a modern of obstetrics and gynecology current from problems It is considered. In treatment pathogenetic approach we this in the article illuminating we gave. This on purpose wide comprehensive literature comment transferred and hormonal and metabolic status correction through to treat this pathology directed research quoted.
Keywords: Polycystic ovaries syndrome, Metabolic syndrome, Insulin resistance, Hyperandrogenism, Lipids exchange, Treatment
Cite this paper: Kh. B. Sattaraliyeva, Method of Improving the Training Treatment Program in Polycystic Ovary Syndrome, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 587-590. doi: 10.5923/j.ajmms.20251503.21.
1. Introduction
- Currently, the main cause of the development of polycystic ovary syndrome (PCOS) is unknown, so therapeutic approaches are symptomatic. The number of potentially effective drugs proposed is sufficient Our knowledge of the true causes of PCOS is as limited as it is extensive. The success of any form of PCOS therapy depends on the treatment used, and sometimes on the characteristics of the population in which the therapy is administered [7,12].The goal of PCOS therapy: Reducing the level of circulating androgens and reducing the clinical manifestations of hyperandrogenism (HA); improving reproductive function and fertility; reducing body weight in the presence of obesity; eliminating complications associated with PCOS and hyperinsulinemic insulin resistance (IR), such as glucose intolerance, dyslipidemia, arterial hypertension, atherosclerosis, and, most importantly, IUGR [3,12].First Goal: to reduce circulating androgen levels and reduce the clinical manifestations of HA. In PCOS, HA is caused by hyperinsulinemic IR, so reducing circulating insulin leads to a reduction in the clinical manifestations of HA [11]. To achieve this goal, drugs are used that are directed at:1) reducing the production of androgens (combined oral contraceptives containing dropirenon, cyproterone acetate; gonadotropin - releasing hormone agonists including drugs such as triptorelin, goserelin, buserelin, leuprorelin; ketoconazole),2) peripheral blockade of the effect of androgens (cyproterone Acetate and spironolactone; flutamide, finasteride - directly antiandrogens, not licensed for use by women) [1,8,10].Second goal: reproduction v function and improve fertility.Hyperinsulinemia affects the IR at many levels - the pituitary gland, ovaries, peripheral metabolism of androgens - and causes chronic anovulation and infertility in women with PCOS. Pharmacological treatment aimed at improving insulin sensitivity helps to reduce its level and leads to spontaneous ovulation or improves standard ovulation induction schemes. To achieve this goal, antiandrogen±estrogen - progestin drugs are used for women with a normal body mass index and without GI. For patients with excess body weight and GI, - Insulin sensitizers are used in conjunction with weight management measures [4].Biochemical evaluation before and after treatment showed positive changes.
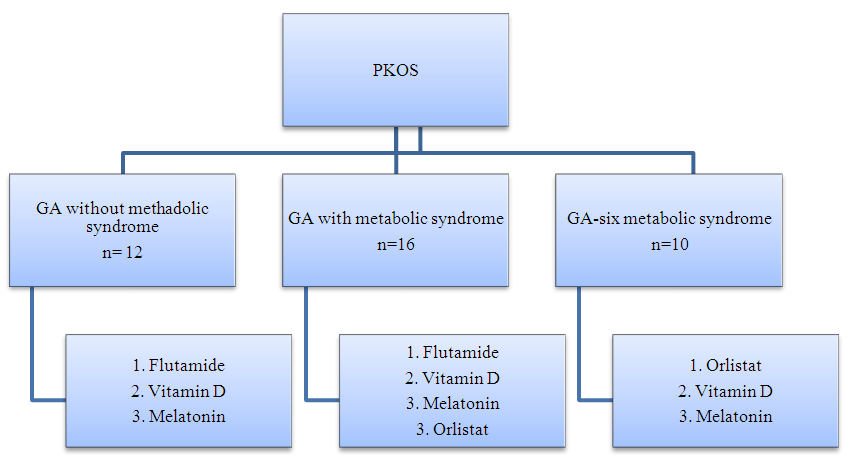 | Figure 1. Algorithm of the preparatory stage for the main group before the EK program |
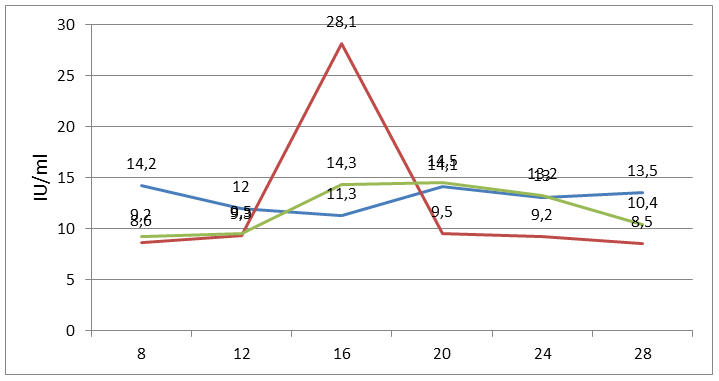 | Figure 2. Changes in the level of LG during the menstrual cycle |
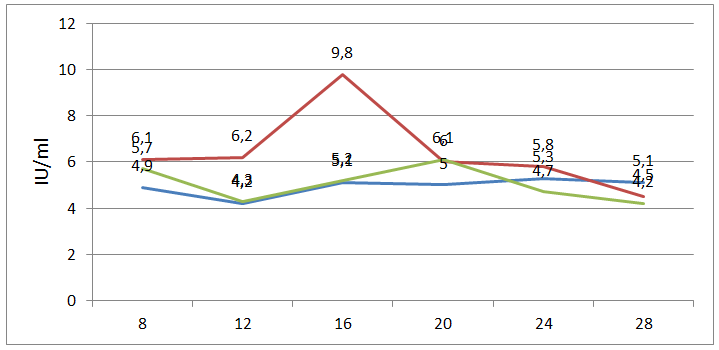 | Figure 3. Changes in FSG levels during the menstrual cycle |
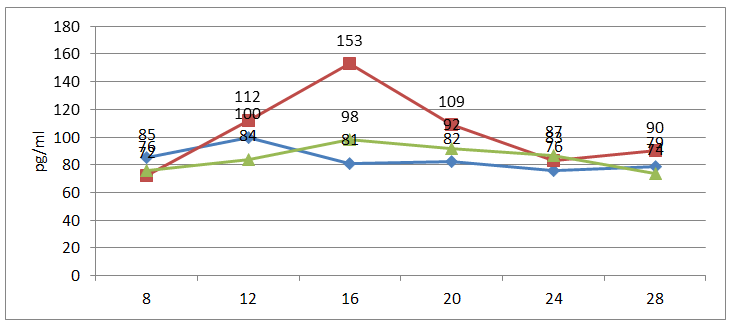 | Figure 4. Changes in estradiol levels during the menstrual cycle |
2. Conclusions
- 1. As a result of the treatment during the preparatory phase, women in the main group tended to have a higher number of oocytes retrieved (15.0 vs. 13.0; P <0.001) and a higher number of fertilizations (12.0 vs. 10.0; P <0.001), as well as a higher number of embryos transferred (2.2 vs. 2.4; P <0.001). 2. A higher IVF rate (80.5% vs. 75.3%; P = 0.003) and a higher number of cycles with quality embryos (97.6% vs. 95.0%; P = 0.002) were observed. Embryo type (cleavage stage embryo, or blastocyst) was not significantly different in both groups (R =0.430). The frequency of implantation, the frequency of clinical pregnancy, and the frequency of childbirth were significantly higher in the main group than in the control group (respectively, 49.3% vs. 38.1%, 70.9% vs. 59.8%, and 58.3% vs. 52.1%; R < 0.001).3. The developed improved program, unlike traditional measures that are long and phased, ensures the restoration of social and physical activity, increases the effectiveness of treatment and rehabilitation by 14%, and socio-economic efficiency by 65%.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML