-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(3): 581-586
doi:10.5923/j.ajmms.20251503.20
Received: Feb. 8, 2025; Accepted: Feb. 25, 2025; Published: Mar. 8, 2025

Diagnostic Markers of Placental Insufficiency in Pregnant Women with Thrombophilia
Safayeva Shirinkhon Furkatovna1, Usmanova Durdona Dzhurabaevna2
1Applicant, Department of Clinical Laboratory Diagnostics, Center for Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
2MD, PhD, Associate Professor, Department of Neurology, Child Neurology and Medical Genetics, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

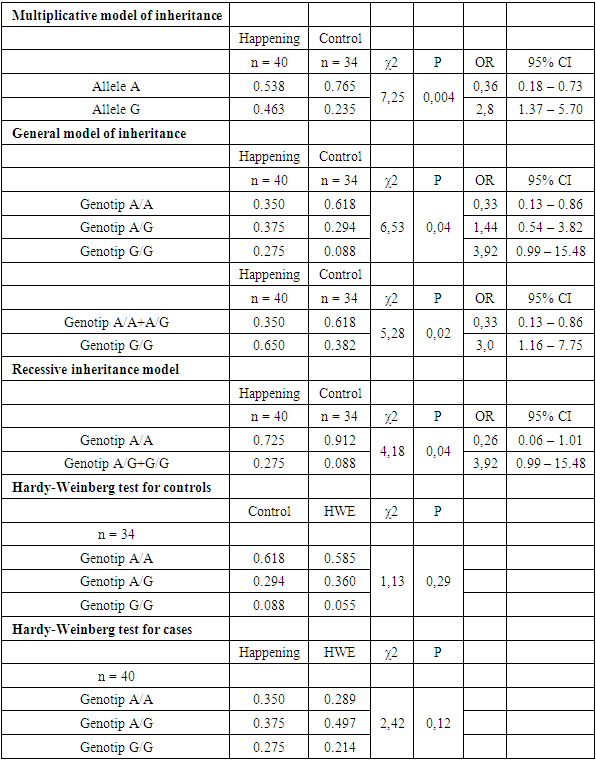
The article describes the results of a molecular genetic study of the hemostasis system with the determination of the polymorphism of the folate-dependent gene (MTHFR methylenetetrahydrofolate reductase: MTHFR1298 A>C MTHFR 677 C>T in the examined pregnant women. Genetic polymorphism 2756 A>G of the MTR gene is associated with the pathology under study in the Uzbek population (χ2=7.25; P=0.004; OR=2.8, CI=1.37–5.70), where the reference allele G has a damaging effect, and the alternative allele A has a protective effect. The presence of the G allele can increase the risk of developing diseases associated with impaired folate metabolism, which makes it an important marker for genetic screening and the development of personalized therapeutic strategies.
Keywords: Marker, Placental insufficiency, Pregnant women, Thrombophilia
Cite this paper: Safayeva Shirinkhon Furkatovna, Usmanova Durdona Dzhurabaevna, Diagnostic Markers of Placental Insufficiency in Pregnant Women with Thrombophilia, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 581-586. doi: 10.5923/j.ajmms.20251503.20.
Article Outline
1. Introduction
- Despite advances in medicine, thrombophilia remains a serious problem for many pregnant women, causing undesirable consequences [1,2].Studies show that thrombophilia and genetic changes in certain genes that regulate blood clotting may be associated with the development of fetoplacental insufficiency. In particular, a link was found between changes in genes responsible for folic acid metabolism and the development of fetoplacental insufficiency in women [3,5,7].However, such studies have not been conducted among Uzbek women with fetoplacental insufficiency, although it is known that they may have increased MTHFR gene polymorphism due to dietary characteristics [4,8,9,10].The results of the study demonstrate that genetic changes associated with metabolism involving folic acid play an important role in the development of fetoplacental insufficiency.The aim of the study: to conduct a molecular genetic study of the hemostasis system with the determination of the polymorphism of the folate-dependent gene (MTHFR methylenetetrahydrofolate reductase: MTHFR1298 A>C MTHFR 677 C>T MTRR 2756 A>G, MTRR 66 A> G in the examined pregnant women.
2. Material and Methods of the Study
- The study included 100 pregnant women who were divided into 2 groups. The main group included 50 pregnant women with placental insufficiency against the background of thrombophilia. The comparison group consisted of 50 pregnant women with placental insufficiency without thrombophilia. The control group consisted of 30 women with a physiological course of pregnancy. All 100 examined women underwent a molecular genetic study revealing hereditary disorders of the hemostasis system: polymorphism of the folate-dependent gene (MTHFR methylenetetrahydrofolate reductase: MTHFR1298 A>C MTHFR 677 C>T MTRR 2756 A>G, MTRR 66 A>G. Genomic DNA was extracted from blood samples using the GeneMATRIX blood DNA purification kit (EURx, Poland). Pre-validated TaqMan real-time PCR methods (Life Technologies, USA) were used to detect the corresponding SNPs in the MTHFR (rs1801131, rs1801133), MTR (rs1805087), and MTRR (rs1801394) genes. Amplification was performed in a 7500 Fast Real-Time PCR System with built-in SDS SNP Genotyping Software (Applied Biosystems, USA) using TaqMan GTXpress Master Mix (Life Technologies, USA).
3. Results of the Study
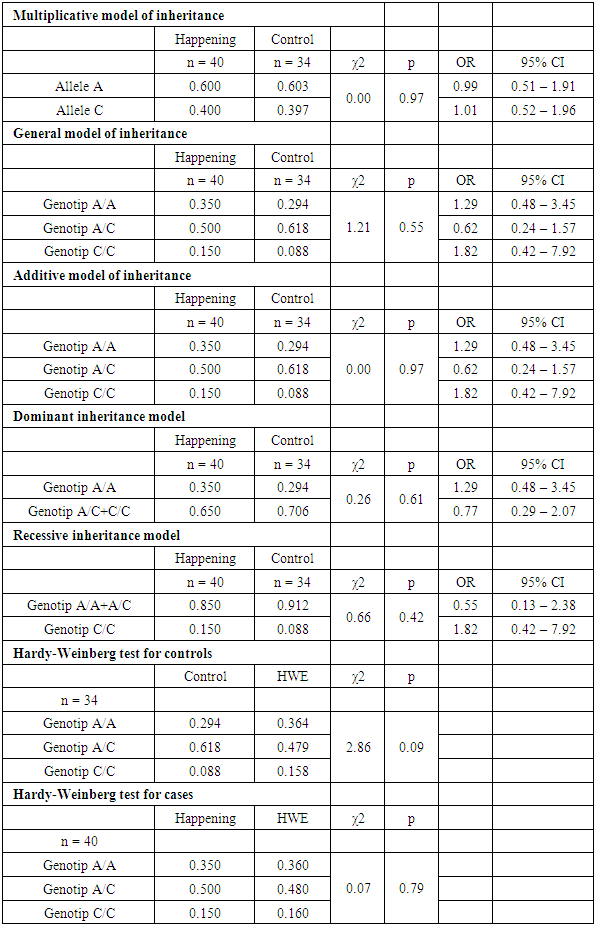
- Analysis of the distribution of allele and genotype frequencies of the polymorphic marker A1298C of the MTHFR gene showed a significant predominance of the heterozygous genotype AC in both groups. The frequency of the reference allele A was 60%, the frequency of the minor allele C - 40% in both study groups. The distribution of allele frequencies in the Uzbek population was slightly different from the world values, so the frequency of the reference allele A averaged in all populations was 76.66%. The frequencies of genotypes in the two groups we compared differed, so in the case group, the frequency of the AA genotype was 35% in the case group, and 29% in the control group. The frequency of the heterozygous genotype also differed in the two groups: the frequency of the AC genotype was 50% in the case group and 62% in the control group (Fig. 1). The distribution of allele frequencies in both groups corresponded to the Hardy-Weinberg distribution, in the case group the value p=0.79, in the control group p=0.09. The association analysis of the influence of the predictor genotype on the occurrence of pathology did not show a significant association for any of the inheritance models used (p>0.05) (table 1).
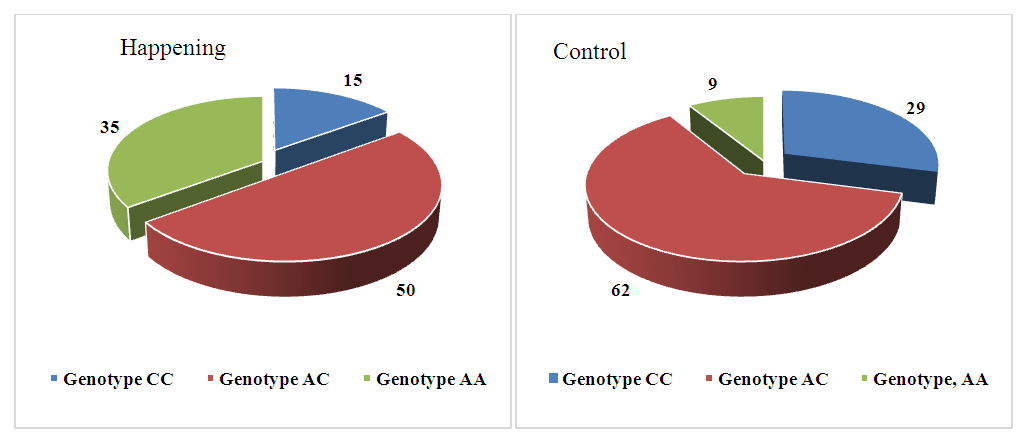 | Figure 1. Distribution of genotype frequencies of the polymorphic marker A1298C of the MTHFR gene in the case and control groups |
|
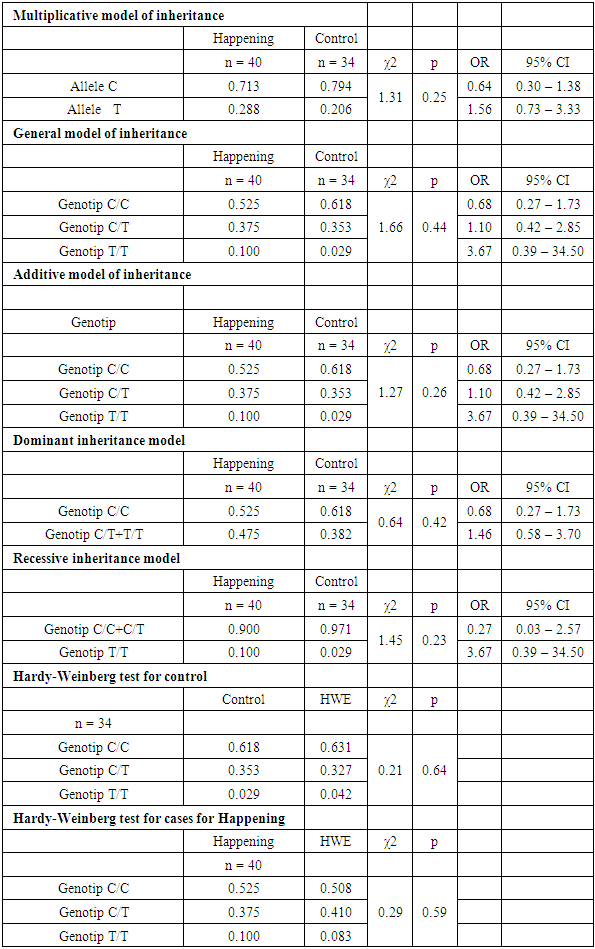
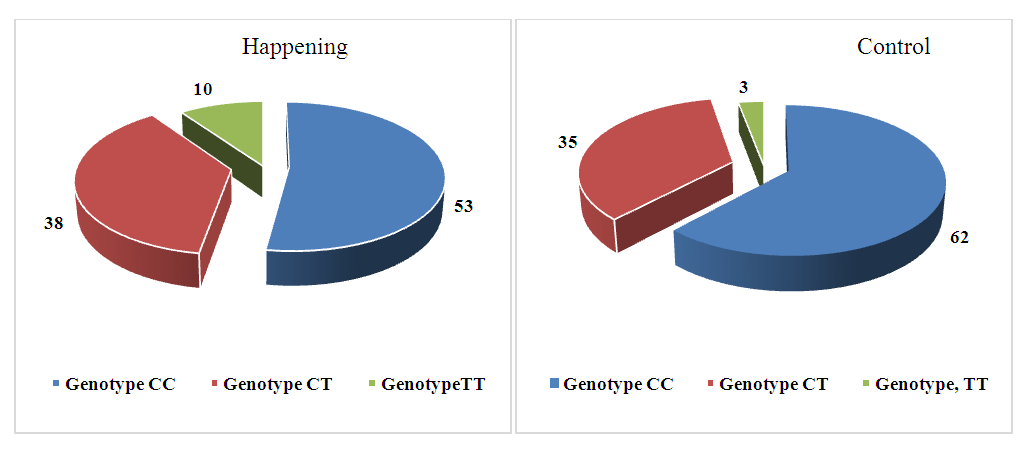 | Figure 2. Distribution of genotype frequencies of the polymorphic marker C677T of the MTHFR gene in the case and control groups |
|
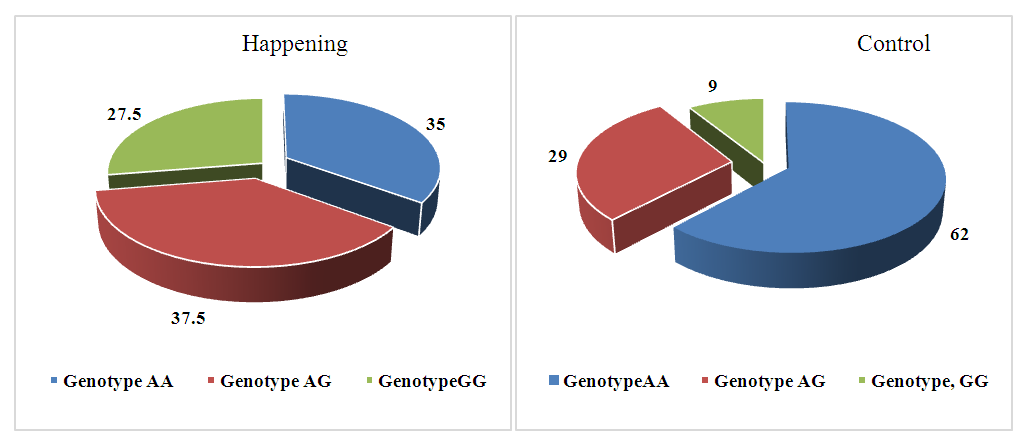 | Figure 3. Distribution of genotype frequencies of the polymorphic marker A2756G of the MTR gene in the case and control groups |
|
4. Conclusions
- Genetic polymorphisms 1298 A>C and 677 C>T of the MTHFR gene, 66 A>G of the MTRR gene, 20210 G>A of the F2 gene, 807 C>T of the α2 ITGA2 gene did not show an association with pathology among the studied women of the Uzbek population according to any of the inheritance models used (p> 0.05).Genetic polymorphism 2756 A>G of the MTR gene is associated with the studied pathology in the Uzbek population (χ2 = 7.25; P = 0.004; OR = 2.8, CI = 1.37–5.70), where the reference allele G has a damaging effect, and the alternative allele A has a protective effect. The presence of the G allele may increase the risk of developing diseases associated with impaired folate metabolism, making it an important marker for genetic screening and the development of personalized therapeutic strategies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML