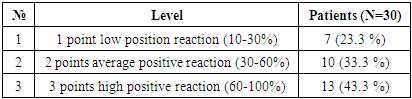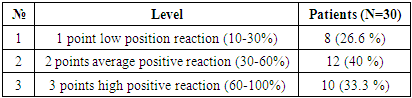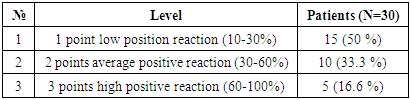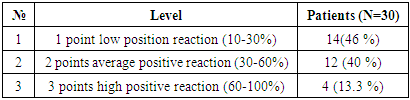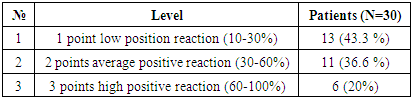Musaboev E. I.1, Abdikhakimov A. N.2, Mirzaev Kh. M.3, Gafur-Akhunov M. A.3, Nishanov D. A.4, Yigitaliyev A. B.5
1Republican Specialized Scientific and Practical Medical Center of Virology, Tashkent, Uzbekistan
2Tashkent Regional Branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology, Tashkent, Uzbekistan
3Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
4Republican Specialized Scientific and Practical Medical Center of Pathological Anatomy, Tashkent, Uzbekistan
5Fergana Medical Institute of Public Health, Fergana, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article is devoted to immunohistochemical studies associated with primary liver cancer (hepatocellular carcinoma - HCC). The topic is relevant, since HCC is one of the aggressive tumors with a high mortality rate, arising as a result of chronic hepatitis B and C, alcoholic or non-alcoholic fatty liver diseases. The study included 60 patients examined in 2020-2023, half of whom were diagnosed with viral hepatitis B or C, and the rest developed HCC without hepatitis. In the immunohistochemical study, molecular genetic markers (Ki-67, Bcl-2, VEGF, and p53) were studied. According to the results, in cases of HCC associated with viral hepatitis, high proliferative activity (Ki-67 >20%), apoptosis index (Bcl-2), angiogenesis activity (VEGF), and gene-suppressor mutations (p53) were observed more often. In cases of HCC without hepatitis, immunohistochemical parameters were revealed at a relatively low level. Molecular-genetic markers in primary liver cancer are important in assessing the aggressiveness of the tumor, the degree of metastasis, and the response to treatment. The research results open up new possibilities for improving the diagnosis, prognosis, and personalized therapy strategies for HCC.
Keywords:
Hepatocellular carcinoma, Ki-67, Bcl-2, VEGF, p53 gene suppressor, Immunohistochemical examination
Cite this paper: Musaboev E. I., Abdikhakimov A. N., Mirzaev Kh. M., Gafur-Akhunov M. A., Nishanov D. A., Yigitaliyev A. B., Results of Immunogystochemical Examination in Primary Liver Cancer, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 554-561. doi: 10.5923/j.ajmms.20251503.14.
1. Introduction
Primary liver cancer (hepatocellular carcinoma, HCC) is one of the most aggressive and fatal malignant tumors. The development of this tumor is associated with chronic liver diseases, including: viral hepatitis B and C infections, alcoholic liver disease, non-alcoholic fatty liver disease [1,2].In recent years, molecular genetic markers that allow predicting the course of the disease, the likelihood of metastasis, and the response to therapy have been actively studied. The most important markers in primary liver cancer are Ki-67, Bcl-2, VEGF, and p53. These biomarkers play an important role in the mechanisms of: tumor cell proliferation, formation of new blood vessels (angiogenesis), cell death (apoptosis), which is very important for determining their diagnosis, prognosis, and treatment tactics [3,4,5].Ki-67 - a marker of tumor proliferation activity - is a protein expressed in all phases of the cell cycle (except for the G0 phase) and is an important marker of the degree of cell proliferation. High Ki-67 expression is associated with tumor aggressiveness, rapid growth, and a poor prognosis [6]. Studies show that high Ki-67 expression in HCC is associated with: high mitotic activity, rapid progression (development), low-grade differentiated tumors, shortened patient lifespan [7,8], high Ki-67 levels can be an independent prognostic marker in the late stages of the disease [9]. Patients are classified based on Ki-67 expression: patients with high Ki-67 levels require aggressive treatment methods, including targeted therapy and systemic treatment [10].Bcl-2 - a protein that regulates apoptosis (B-cell lymphoma 2) - is an anti-apoptotic protein that prevents apoptosis (natural cell death). Disruption of apoptosis leads to the survival of cancer cells [11]. Studies show that Bcl-2 expression was detected in 40-60% of HCC cases. Its high level is associated with: resistance of cancer cells to apoptosis, low sensitivity to chemotherapy, and a high risk of relapse [12,13]. Determination of the Bcl-2 level allows predicting the sensitivity of the tumor to treatment. Bcl-2 inhibitors (for example, venetoclax) are considered promising drugs in the treatment of HCC [14].VEGF - angiogenesis factor (vascular endothelial growth factor) - the main protein regulating angiogenesis, stimulating the formation of new blood vessels for the blood supply of the tumor [15]. High VEGF expression is associated with: active angiogenesis, high vascularization (blood supply) of the tumor, a high risk of metastasis, and a shorter patient lifespan [16,17]. Anti-VEGF therapy (bevatsizumab, ramutsirumab) is used in the treatment of HCC and improves the prognosis in patients with high levels of VEGF [18].p53 - tumor-suspending protein - is a protective protein against tumors that controls the cell cycle and regulates apoptosis. p53 mutations lead to uncontrolled cell proliferation and cancer development [19]. p53 mutations have been detected in 25-50% of HCC cases. impaired p53 function is associated with tumor aggressiveness, therapy resistance, and the risk of metastasis [20]. determining p53 levels helps predict treatment response. MDM2 inhibitors (negative regulators of p53) are considered as promising targeted therapy for HCC [21].Thus, molecular genetic markers Ki-67, Bcl-2, VEGF, and p53 play an important role in the pathogenesis and development of hepatocellular carcinoma (HC). Their study will allow improving the diagnosis of the disease and predicting its course, choosing personalized therapy, and improving the development of new targeted drugs for HCC: [18,19,20]. Further study of these biomarkers is necessary for the early detection of primary liver cancer and the development of effective treatment strategies.
2. Materials and Methods of Research
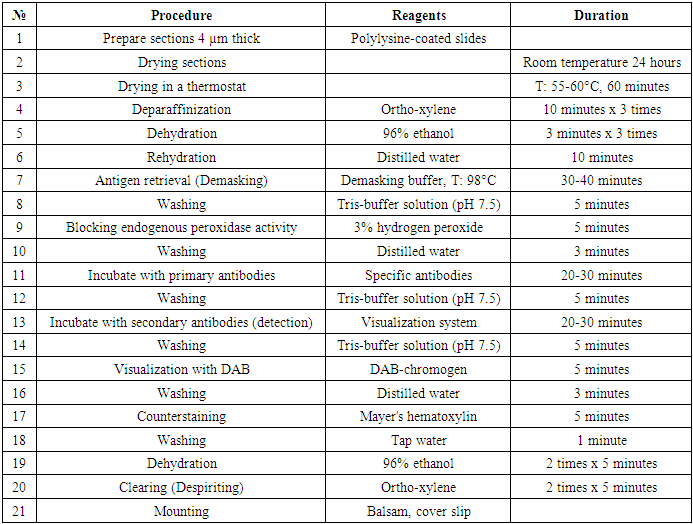
In the study, 60 patients with primary liver cancer examined and treated at the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology and its Tashkent regional branch were examined retrospectively and prospectively in 2020-2023, and an immunohistochemical examination method was conducted, in which 30 (50%) patients had a history of viral hepatitis B and C and were treated. Later, after a chronic illness, he was diagnosed with primary hepatocellular carcinoma and hospitalized. In 30 (50%) patients, viral hepatitis was not detected in the medical history, but subsequently, hepatocellular liver cancer was diagnosed and treated. The results of histological examination and immunohistochemical studies of these patients were studied. From a pathomorphological point of view, the use of the immunohistochemical method in hepatocellular liver cancer, in which the study of molecular genetic markers is carried out for the first time, reveals its more morphologically significant features, plays a special role in determining treatment tactics and predicting the disease.It is known from the literature that currently immunohistochemical examination is recognized as the gold standard in the world among morphological studies in clinical oncology. Biopsies (trepan-biopsy, surgical material) obtained from all patients were taken from paraffin blocks, processed for immunohistochemical examination, and examined by taking sections on a slide. By immunohistochemical examination, the Ki67, bcl 2, VHFR, and w p53 genes were studied. The conducted immunohistochemical examination method was technically carried out as follows (Table 1).Table 1. Stages of immunohistochemical (IHC) examination
 |
| |
|
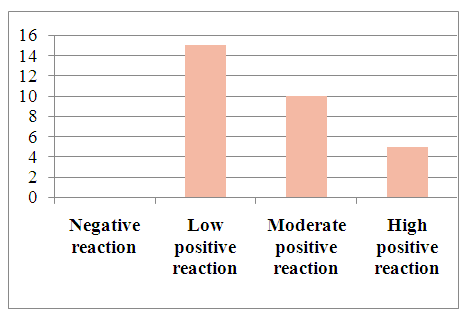
Pathomorphologically, hepatocellular liver cancer was studied by the immunohistochemical method in a total of 60 patients. For immunohistochemical examination, the expression of Ki67, Vcl2, VGFR and w p53 monoclonal antibodies in cells was studied using a Bond Leica Australia (Australia) immunohistoprocessor.In our study, the expression of molecular structures in liver cancer cells was studied in two groups, that is, in patients of the first group, the development of hepatocellular carcinoma caused the development of viral hepatitis B, C, while in patients of the second group, liver cancer was not caused by viral hepatitis.30 patients with hepatocellular carcinoma developed as a result of viral hepatitis B, C were selected. In the study, different results were obtained when studying the markers Ki67, Vcl2, VHFR, and w p53 in liver cancer cells.Results: The Ki67 indicator morphologically characterized the staining of the cell nucleus in hepatocellular liver cancer as follows: the obtained results were assessed as a mild, moderate, and severe positive reaction. The study results show that out of 30 patients, 5 (16.6%) had a mild positive reaction, 10 (33%) had a moderate positive reaction, and 15 (50%) had a high positive reaction. No negative reaction processes were observed (Table 2).Table 2. Ki67 indicators in hepatocellular carcinoma associated with viral hepatitis B, C
 |
| |
|
Microscopically, liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae are not preserved, malignant tumor cells form tumor islands of polymorphic hepatocytes with pathological mitosis and cell necrobiosis. The nuclei of tumor cells are stained dark brown (Fig. 1).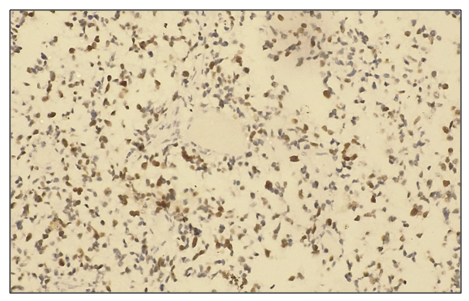 | Figure 1. A high degree of positive response to the Ki 67 marker in patients diagnosed with hepatocellular carcinoma, viral hepatitis B (80%). IHC - Dab chromogen. Ob10. Ok40 |
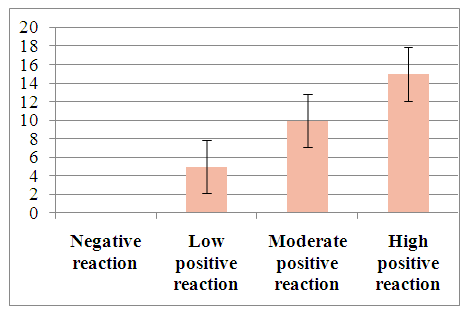 | Figure 2. Degree of proliferative activity of tumor cells when hepatocellular carcinoma develops against the background of viral hepatitis B |
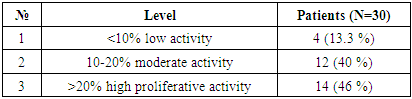
To determine tumor apoptosis in hepatocellular carcinoma with viral hepatitis B, the Vcl2 indicator was studied in 30 patients. In patients, the Vcl2 marker was used to determine tumor apoptosis, which regulates cell death by controlling the permeability of the mitochondrial membrane. The obtained results were evaluated using the ALLRED method. It looks at how many percent of the system's cells are positive for their receptors and how well the receptors appear after staining. These data are then combined to evaluate the sample on a scale from 1 to 3. In this case, the minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 4 (13.3%) had a mild positive reaction, 12 (40%) had a moderate positive reaction, and 14 (46%) had a high positive reaction (Table 3).Table 3. Positive reaction of the Bcl-2 marker in patients with viral hepatitis B,C in hepatocellular carcinoma
 |
| |
|
Microscopically: liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae are not preserved, malignant tumor cells form tumor islands of polymorphic hepatocytes with pathological mitosis and cell necrobiosis. The cell membranes of malignant tumors are stained dark brown.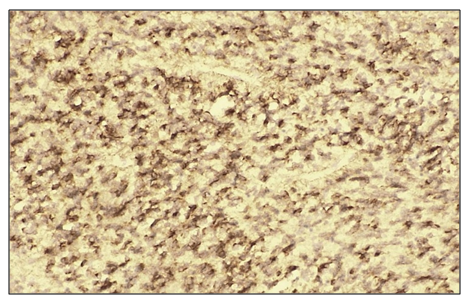 | Figure 3. High positive reaction of the Bcl-2 marker in patients diagnosed with viral hepatitis B in hepatocellular carcinoma. (70%). IHC - Dab chromogen. Ob10. Ok40 |
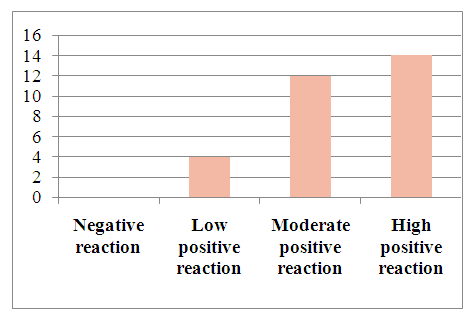 | Figure 4. Level of Bcl-2 marker expression in patients diagnosed with viral hepatitis B in hepatocellular carcinoma |
The results of w p53 expression in hepatocellular carcinoma with viral hepatitis B were assessed using the ALLRED method. It considers what percentage of system cells are positive for receptors and how well receptors appear after staining. Then these data were combined for a sample assessment on a scale from 1 to 3. The minimum score was 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 7 (23.3%) had a mild positive reaction, 10 (13.3%) had a moderate positive reaction, and 13 (43.3%) had a high positive reaction (Table 4).Table 4. Level of expression of the p53 marker in hepatocellular carcinoma against the background of viral hepatitis C
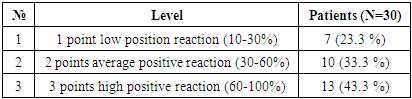 |
| |
|
When viewed under a microscope, liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae are not preserved, malignant tumor cells form tumor islands of polymorphic hepatocytes with pathological mitosis and cell necrobiosis. The nuclei of malignant tumor cells are stained dark brown (Fig. 3).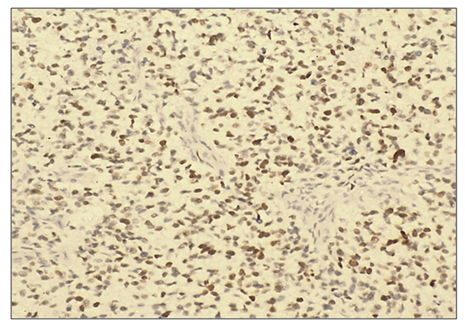 | Figure 5. High positive reaction of the p53 marker in patients diagnosed with viral hepatitis B in hepatocellular carcinoma. (90%). IHC - Dab chromogen. Ob10. Ok40 |
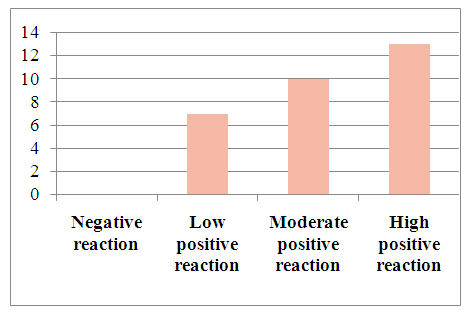 | Figure 6. Level of the p53 marker in patients diagnosed with viral hepatitis C in hepatocellular carcinoma |
In detected hepatocellular carcinoma of viral hepatitis C. The obtained results were considered in what percentage of cells the markers were positive and how expressive they were after staining. These data were then combined to evaluate the sample on a scale from 1 to 3. The minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 8 (26.6%) had a mild positive reaction, 12 (40%) had a moderate positive reaction, and 10 (33.3%) had a high positive reaction (Table 5).Table 5. Level of expression of the HFR marker in patients with viral hepatitis C in hepatocellular carcinoma
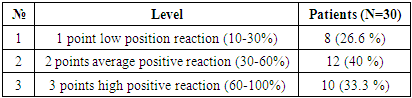 |
| |
|
Microscopically: liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae not preserved, malignant tumor cells form tumor islands with pathological mitosis of polymorphic hepatocytes and cell necrobiosis. The endothelium of blood vessels is stained dark brown. | Figure 7. High positive reaction of the VHFR marker in patients diagnosed with viral hepatitis B in the light cell type of hepatocellular carcinoma. (90%). IHC - Dab chromogen. Ob10. Ok40 |
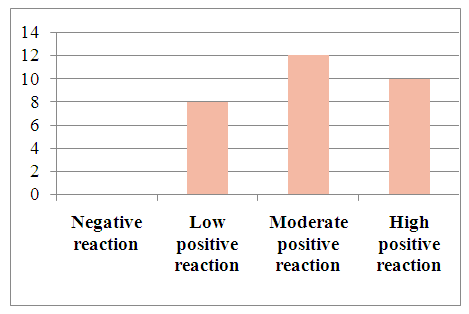 | Figure 8. The level of the VHFR marker in patients with viral hepatitis C in hepatocellular carcinoma |
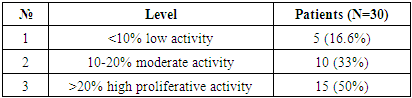
For the purpose of studying patients with hepatocellular carcinoma, in whom viral hepatitis was not diagnosed, 30 patients were selected. The results obtained in all patients were evaluated as a percentage of the marker of proliferative activity of Ki 67 tumor cells. The staining of nuclear cells was characterized as follows: <10% - low activity, 10-20% - moderate activity, >20% - high proliferative activity. Through these results, it is possible to determine the prognostic factor of cancer. The obtained results were assessed as mild, moderate, and severe positive reactions. Of the 30 observed patients, 15 (50%) had a mild positive reaction, 10 (33.3%) - a moderate positive reaction, and 5 (16.6%) - a high positive reaction. No negative reaction processes were observed (Table 6).Table 6. Level of proliferative activity of the Ki 67 marker in patients with hepatocellular carcinoma without the detection of viral hepatitis
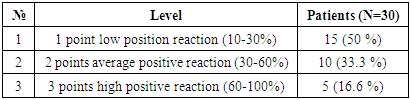 |
| |
|
Microscopically: liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae not preserved, malignant tumor cells form tumor islands, pathological mitosis of polymorphic hepatocytes and cell necrobiosis. The nuclei of malignant tumor cells are stained dark brown. It is characterized by a low level of proliferative activity of malignant tumor cells.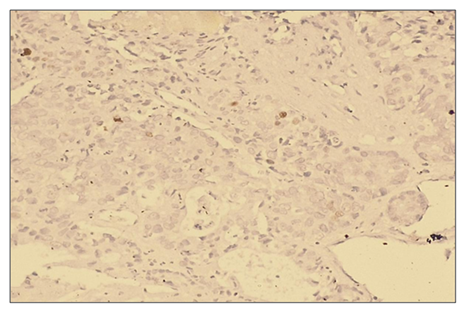 | Figure 9. Low positive reaction of the Ki 67 marker in patients with hepatocellular carcinoma without viral hepatitis (50%). IHC - Dab chromogen. Ob10. Ok40 |
 | Figure 10. Degree of proliferative activity of the Ki 67 marker in patients with hepatocellular carcinoma without viral hepatitis |
For the purpose of determining tumor apoptosis in hepatocellular carcinoma, in which viral hepatitis was not detected, 30 patients were selected. In patients, the Bcl-2 marker is used to detect tumor apoptosis, which regulates cell death by controlling the permeability of the mitochondrial membrane. The obtained results were evaluated using the ALLRED method. The system looks at what percentage of cells are positive for their receptors and how well the receptors appear after staining. These data are then combined to evaluate the sample on a scale from 1 to 3. In this case, the minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 14 (46%) had a mild positive reaction, 12 (40%) had a moderate positive reaction, and 4 (13.3%) had a high positive reaction. (Table 7).Table 7. Positive reaction of the Bcl-2 marker in 30 patients with hepatocellular carcinoma without the detection of viral hepatitis
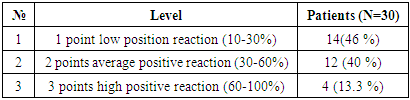 |
| |
|
Microscopically: liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae are not preserved, malignant tumor cells form tumor islands, pathological mitosis of polymorphic hepatocytes and cell necrobiosis. The membranes of malignant tumor cells are stained dark brown (Fig. 11).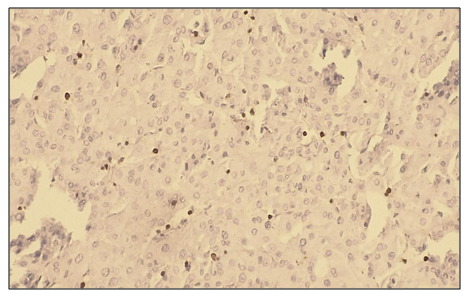 | Figure 11. Low positive reaction of the Bcl-2 marker in patients with hepatocellular carcinoma without viral hepatitis. (30%). IHC - Dab chromogen. Ob10. Ok40 |
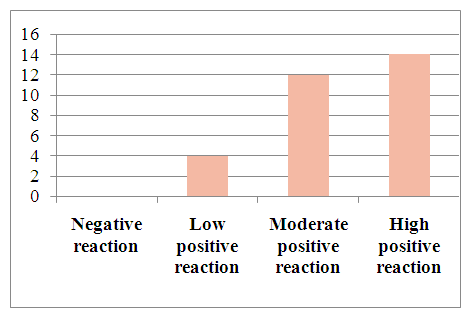 | Figure 12. Expression of the Bcl-2 marker in patients with hepatocellular carcinoma without viral hepatitis |
In immunohistochemical studies of the p53 tumor suppressor gene in hepatocellular carcinoma without viral hepatitis, the data were combined to assess the samples on a scale from 1 to 3. The scoring system was as follows: 0 (negative), 1 point (low positive 10-30%), 2 points (moderate positive 30-60%), 3 points (high positive 60-100%). Among the 30 selected patients, 13 (43.3%) showed a mild positive reaction, 11 (36.6%) showed a moderate positive reaction, and 6 (20%) showed a high positive reaction. (See Figure 13).Table 8. Positive reaction of the p53 marker in patients with hepatocellular carcinoma without viral hepatitis
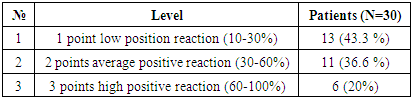 |
| |
|
Microscopically: liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae are not preserved, malignant tumor cells form tumor islands of polymorphic hepatocytes with pathological mitosis and cell necrobiosis. The nuclei of malignant tumor cells are stained dark brown.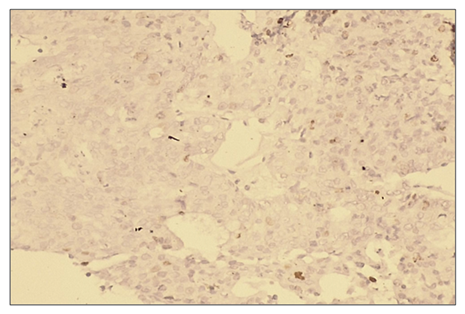 | Figure 13. Low positive reaction of the p53 marker in patients with hepatocellular carcinoma without viral hepatitis. (20%). IHC - Dab chromogen. Ob10. Ok40 |
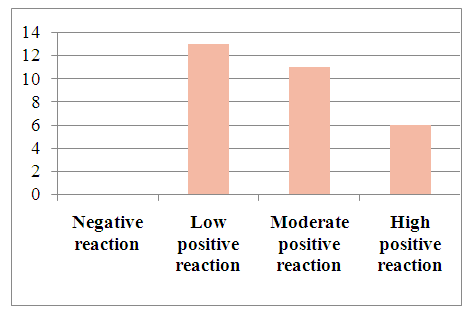 | Figure 14. Expression of p53 marker in patients with hepatocellular carcinoma without detected viral hepatitis |
When studying the vascular endothelial growth factor (VEGF) by immunohistochemical method in hepatocellular carcinoma without viral hepatitis, the data are combined to assess the sample on a scale from 1 to 3. In this case, the minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, a mild positive reaction was detected in -, a moderate positive reaction in -, and a high positive reaction in -. (Table 9).Table 9. Positive reaction of the VGFR marker in patients with hepatocellular carcinoma without detected viral hepatitis
 |
| |
|
Microscopically: liver cells with hyperplasia and polymorphism of hepatocytes, trabeculae are not preserved, malignant tumor cells form tumor islands of polymorphic hepatocytes with pathological mitosis and cell necrobiosis. The endothelium of blood vessels is stained dark brown.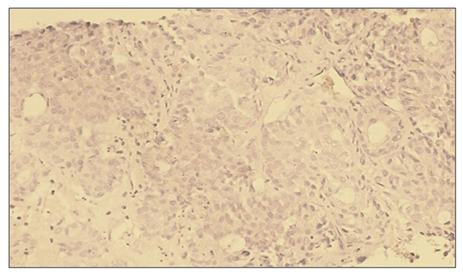 | Figure 15. Low positive reaction of the VHFR marker in patients with hepatocellular carcinoma without viral hepatitis. IHC - Dab chromogen. Ob10. Ok40 |
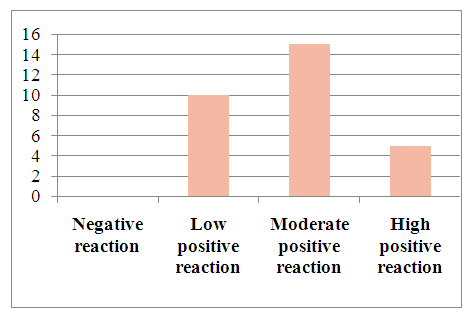 | Figure 16. VGFR marker levels in patients with hepatocellular carcinoma without detected viral hepatitis |
3. Conclusions
In our study, 30 patients were selected for the purpose of examining patients with viral hepatitis B, C diagnosed with hepatocellular carcinoma. The results obtained in all patients were evaluated as a percentage of the marker of proliferative activity of Ki 67 tumor cells. The staining of nuclear cells is described as follows. <10% low activity, 10-20% moderate activity, >20% high proliferative activity. Based on these results, it is possible to determine the prognostic factor of cancer.The obtained results were assessed as mild, moderate, and severe positive reactions. Of the 30 observed patients, 5 (16.6%) had a mild positive reaction, 10 (33%) had a moderate positive reaction, and 15 (50%) had a high positive reaction. No negative reaction processes were observed.To determine the apoptosis of hepatocellular carcinoma with viral hepatitis B and C, 30 patients were selected. In patients, the Bcl-2 marker is used to detect tumor apoptosis, which regulates cell death by controlling the permeability of the mitochondrial membrane. The obtained results were evaluated using the ALLRED method. The system looks at what percentage of cells are positive for their receptors and how well the receptors appear after staining. These data are then combined to evaluate the sample on a scale from 1 to 3. In this case, the minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 4 (13.3%) had a mild positive reaction, 12 (40%) had a moderate positive reaction, and 14 (46%) had a high positive reaction.In hepatocellular carcinoma diagnosed with viral hepatitis B, it is considered what percentage of the genes-suppressor - p53 system cells are positive for receptors and how well the receptors appear after staining. These data are then combined to evaluate the sample on a scale from 1 to 3. In this case, the minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 7 (23.3%) had a mild positive reaction, 10 (33.3%) had a moderate positive reaction, and 13 (43.3%) had a high positive reaction.VGFR, a signaling protein produced by cells to stimulate angiogenesis in hepatocellular carcinoma with viral hepatitis C, was studied.The obtained data were combined to evaluate the sample on a scale from 1 to 3. In this case, the minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 8 (26.6%) had a mild positive reaction, 12 (40%) had a moderate positive reaction, and 10 (33.3%) had a high positive reaction.For the purpose of conducting a study of patients diagnosed with hepatocellular carcinoma without viral hepatitis, 30 patients were selected. The results obtained in all patients were evaluated as a percentage of the marker of proliferative activity of Ki 67 tumor cells.The obtained results were assessed as mild, moderate, and severe positive reactions. Of the 30 observed patients, 15 (50%) had a mild positive reaction, 10 (33.3%) had a moderate positive reaction, and 5 (16.6%) had a high positive reaction. No negative reaction processes were observed.For the purpose of determining tumor apoptosis in hepatocellular carcinoma without viral hepatitis, 30 patients were selected. In patients, the Bcl-2 marker is used to detect tumor apoptosis, which regulates cell death by controlling the permeability of the mitochondrial membrane. The obtained results were evaluated using the ALLRED method. The system considers what percentage of cells are positive for their receptors and how well the receptors appear after staining. These data are then combined to evaluate the sample on a scale from 1 to 3. In this case, the minimum score is 0 (negative), 1 point (low positive 10-30%), 2 points (medium positive 30-60%), 3 points (high positive 60-100%). Of the 30 selected patients, 14 (46%) had a mild positive reaction, 12 (40%) had a moderate positive reaction, and 4 (13.3%) had a high positive reaction. In hepatocellular carcinoma without viral hepatitis, 13 (43.3%) patients with a mild positive reaction, 11 (36.6%) patients with a moderate positive reaction, and 6 (20%) patients with a high positive reaction were identified.In hepatocellular carcinoma, in which viral hepatitis was not detected, a mild positive reaction was detected in 10 (33.3) of 30 patients, a moderate positive reaction in 15 (50%), and a high positive reaction in 5 (16.6%) patients, who were selected for signaling protein (SBP) produced by cells to stimulate angiogenesis. The obtained results showed that in hepatocellular carcinoma with viral hepatitis B and C, immunohistochemical indicators Ki67, Bcl 2, VHFR and w p53 markers were highly positive. In patients with hepatocellular carcinoma without viral hepatitis, low immunohistochemical indicators of Ki67, Bcl 2, VHFR, and w p53 markers were observed. The obtained results showed that the type of cancer resulting from viral hepatitis B and C has an aggressive course and a high degree of metastasis to neighboring organs.
References
| [1] | Bruix, J., Reig, M., & Sherman, M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology, 2019. 156(2), 411–421. https://doi.org/10.1053/j.gastro.2018.08.065. |
| [2] | Craig, A. J., von Felden, J., Garcia-Lezana, T., Sarcognato, S., & Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nature Reviews Gastroenterology & Hepatology, 2020. 17(3), 139–152. https://doi.org/10.1038/s41575-019-0229-4. |
| [3] | El-Serag, H. B., & Rudolph, K. L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology, 2017. 132(7), 2557–2576. https://doi.org/10.1053/j.gastro.2017.02.063. |
| [4] | Fan, B., Malato, Y., Calvisi, D. F., Sun, H. C., & Wang, X. W. Ki-67 expression as a prognostic marker in hepatocellular carcinoma. BMC Cancer, 2020. 20(1), 1123. https://doi.org/10.1186/s12885-020-07620-2. |
| [5] | Faivre, S., Rimassa, L., & Finn, R. S. Molecular therapies for hepatocellular carcinoma: What can we target? Nature Reviews Clinical Oncology, 2020. 17(7), 409–423. https://doi.org/10.1038/s41571-020-0377-8. |
| [6] | Forner, A., Reig, M., & Bruix, J. Hepatocellular carcinoma. The Lancet, 2018. 391(10127), 1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2. |
| [7] | Guichard, C., Amaddeo, G., Imbeaud, S., & Letouzé, E. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nature Genetics, 2019. 44(6), 694–698. https://doi.org/10.1038/ng.2256. |
| [8] | He, G., & Karin, M. NF-κB and STAT3 – Key players in liver cancer. Cell Research, 2019. 29(8), 574–586. https://doi.org/10.1038/cr.2019.32. |
| [9] | Hoshida, Y., Villanueva, A., Kobayashi, M., Peix, J., & Chiang, D. Y. Gene expression in hepatocellular carcinoma. Cancer Research, 2017. 78(2), 250–257. https://doi.org/10.1158/0008-5472.CAN-17-1725. |
| [10] | Ikeda, M., Sung, M. W., Kudo, M., et al. Atezolizumab plus bevacizumab in hepatocellular carcinoma. New England Journal of Medicine, 2021. 383(24), 2424–2434. https://doi.org/10.1056/NEJMoa1915745. |
| [11] | Kudo, M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2025. Oncology, 2019. 97 (Suppl 1), 131–141. https://doi.org/10.1159/000503045. |
| [12] | Lee, J. S., & Thorgeirsson, S. S. Genetic profiling of hepatocellular carcinoma: Classification and prognosis. Hepatology, 2019. 70(4), 1419–1432. https://doi.org/10.1002/hep.30878. |
| [13] | Lin, D. C., Mayakonda, A., Dinh, H. Q., et al. Genomic and epigenomic heterogeneity of hepatocellular carcinoma. Cancer Research, 2020. 80(10), 1914–1925. https://doi.org/10.1158/0008-5472.CAN-19-1725. |
| [14] | Llovet, J. M., Kelley, R. K., Villanueva, A., et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nature Reviews Clinical Oncology, 2021. 18(10), 595–613. https://doi.org/10.1038/s41571-021-00574-6. |
| [15] | Mak, L. Y., Cruz-Ramón, V., Chinchilla-López, P., et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. The American Journal of Gastroenterology, 2021. 116(3), 457–478. https://doi.org/10.14309/ajg.0000000000001051. |
| [16] | Sherman, M. Hepatocellular carcinoma: Screening and diagnosis. Clinical Liver Disease, 2018. 22(1), 61–74. https://doi.org/10.1002/cld.784. |
| [17] | Sun, H. C., Zhuang, P. Y., Qin, L. X., et al. VEGF and hepatocellular carcinoma angiogenesis. Journal of Cancer Research and Clinical Oncology, 2020. 146(6), 1517–1526. https://doi.org/10.1007/s00432-020-03172-y. |
| [18] | Tang, Z. Y. Hepatocellular carcinoma – Causes, diagnosis, and treatment. The Lancet Oncology, 2018. 19(11), 1300–1312. https://doi.org/10.1016/S1470-2045(18)30337-0. |
| [19] | Villanueva, A. p53 mutations and liver cancer progression. Hepatology, 2021. 74(2), 1047–1056. https://doi.org/10.1002/hep.31783. |
| [20] | Yang, J. D., Hainaut, P., Gores, G. J., et al. A global view of hepatocellular carcinoma: Trends, risk, prevention, and management. Nature Reviews Gastroenterology & Hepatology, 2018. 16(10), 589–604. https://doi.org/10.1038/s41575-019-0186-y. |


















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML