-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(3): 514-523
doi:10.5923/j.ajmms.20251503.06
Received: Jan. 23, 2025; Accepted: Feb. 22, 2025; Published: Mar. 5, 2025

Scoring System for Differential Diagnosis of Tuberculous Spondylitis and Metastatic Lesions of the Spine
Z. P. Makhmudova
Republican Specialized Scientific Research Center of Phthisiology and Pulmonology, named after Sh. A. Alimov, MH RUz, Tashkent, Uzbekistan
Correspondence to: Z. P. Makhmudova, Republican Specialized Scientific Research Center of Phthisiology and Pulmonology, named after Sh. A. Alimov, MH RUz, Tashkent, Uzbekistan.
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

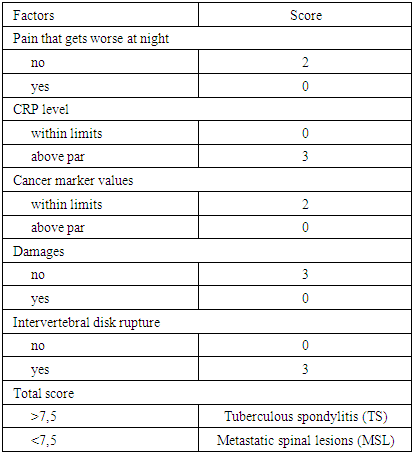
Purpose: Tuberculous spondylitis (TS) and metastatic spinal lesions (MSL) are common diseases with similar clinical and X-ray-CT-MRI manifestations. Often, scientific reports have reported cases of undifferentiated diagnosis of metastatic lesions in spinal tuberculosis (TB), or metastatic lesions in the spine were treated as tuberculous spondylitis. Although pathologic examination is the gold standard for confirming the diagnosis, it is impractical to perform biopsy in all patients. The aim of this study is to establish a scoring system to facilitate the differential diagnosis of tuberculous spondylitis (TS) and metastatic lesions in the spine (MSL) before invasive procedures. Methods: a total of 147 patients were retrospectively analyzed, including 77 with spinal tuberculosis and 70 with ML. These patients underwent inpatient examination in the Department of Bone and Joint Pathology at the Republican Specialized Scientific Research Center of Phthisiology and Pulmonology, named after Sh. A. Alimov, MH RUz, Tashkent, Uzbekistan, from 2020 to 2024. The clinical characteristics that were recorded, registered and analyzed are as follows: age, gender, history of volumetric masses, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, and imaging features, including characteristics of spinal lesion spread, subligamentous spread, para- or prevertebral abscesses, psoas abscesses or presacral abscesses, nature of the affected vertebra and intervertebral disc, presence and formation of sequestrations. The prevalence of clinical features in spinal metastasis was assessed and a scoring system was developed using logistic regression analysis. The effectiveness of the scoring system was also validated prospectively. Results: This scoring system consisted of 5 items: pain worse at night (0 or 2 points), CRP levels (0 or 3 points), oncomarkers (0 or 2 points), lesion foci (0 or 3 points), and intervertebral space destruction (0 or 3 points). Patients with scores greater than 7.5 would otherwise be diagnosed with spinal tuberculosis, ML. According to internal validation, the sensitivity and specificity of the system were 87.9% and 91.6%, respectively. Conclusion: This study developed and validated a scoring system that can be used to differentiate tuberculous spinal lesions from metastatic spinal lesions, which will help clinicians in the rapid and accurate differential diagnosis of both pathologies.
Keywords: Tuberculous spondylitis, Metastatic spinal lesion, Evaluation scoring system, Differential diagnosis
Cite this paper: Z. P. Makhmudova, Scoring System for Differential Diagnosis of Tuberculous Spondylitis and Metastatic Lesions of the Spine, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 514-523. doi: 10.5923/j.ajmms.20251503.06.
1. Introduction
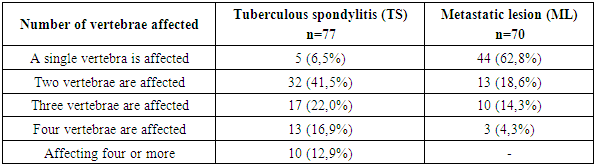
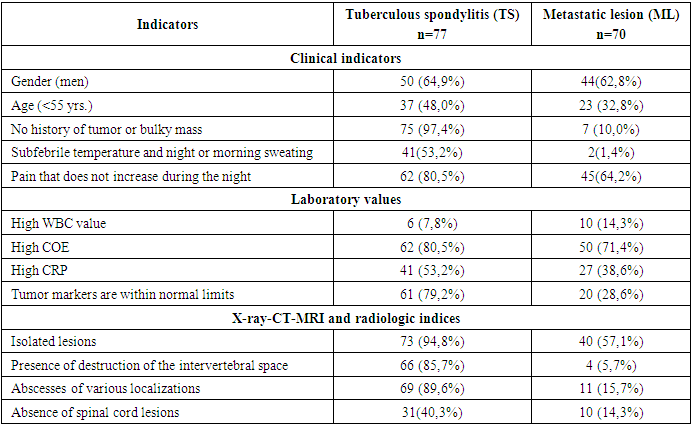
- The epidemiologic indicators of tuberculosis in Uzbekistan have improved significantly in recent years [1].The increase in extrapulmonary manifestations of tuberculosis observed in recent years is associated with the problem of drug resistance of Mycobacterium tuberculosis, which is steadily growing, partly due to the increasing number of patients with primary immunodeficiency (HIV infection, congenital defects of immune protection), as well as the rising number of patients with secondary immunodeficiency resulting from chronic diseases of internal organs and diabetes mellitus. This trend is also linked to improper lifestyle, harmful environmental factors, and the widespread use of antibiotics [2,3].As a consequence, the clinical and radiologic picture of modern tuberculous spondylitis has become diverse, the duration of treatment has increased, and the prognosis has changed.Tuberculosis of extrapulmonary localization, in particular, tuberculosis of bones and joints remains one of the most complex and urgent medical and social problems. Bone and joint tuberculosis occupies the first place in the structure of extrapulmonary tuberculosis morbidity. In the structure of bone and joint tuberculosis, the spine is affected by more than 80% of cases, and widespread, advanced and complicated forms of the process are more frequently detected [5,6,8].Consequently, the clinical manifestation of spinal tuberculosis is marked by diversity, which depends on the age of the patient, the stage of the process, the biological resistance of the organism, the virulence of the pathogen and many other factors. Spinal metastases and spinal tuberculosis are common spinal lesions [1,2], but their treatment methods are quite different. Spinal metastases are malignant lesions, and surgery may be the optimal treatment [3], while spinal tuberculosis is a benign disease, and effective anti-tuberculosis chemotherapy is of great importance [4]. Thus, distinguishing between spinal metastases and tuberculosis is important to reduce pain, prevent neurological disability, minimize spinal deformity, and improve prognosis [5,6]. However, spinal metastases and spinal tuberculosis share similar clinical manifestations and imaging features, such as spinal lesions pain, weakness, weight loss, vertebral destruction, pathological fractures, kyphosis deformity and even neurological impairment [7], so it is difficult to accurately distinguish them from each other, especially in the outpatient department, due to limited consultation time and examination conditions [8]. Although biopsy has been shown to be the gold standard to distinguish spinal metastases from spinal tuberculosis [9], it cannot be performed in the outpatient setting, so in actual outpatient workup, the diagnosis has mainly depended on a combination of clinical findings and ancillary examination [3,10]. However, since not everyone has typical clinical signs of spinal metastasis or spinal tuberculosis, false diagnosis of spinal metastasis has often been reported, even during hospitalization [11-14]. Even worse, incorrect outpatient diagnosis can negatively affect patients' treatment choices [15,16]. Nevertheless, metastasis remains the leading cause of death in the majority of breast cancer patients and represents a major obstacle to reducing mortality from advanced breast cancer, which impair quality of life and reduce overall survival of breast cancer patients [16,18,19].The spine is a frequent site of metastatic lesions, as up to 70% of patients with malignant neoplasms have metastases to the spine at autopsy [8,14,17]. And metastases account for 96% of all spinal tumors [28,29].The risk of metastases to the spine increases with age, the time since the diagnosis and the number of accompanying diseases.Cancer cells can metastasize to the spine in a variety of cancers, including breast cancer, myeloma, cervical cancer, basal cell cancer, peripheral cholangiocarcinoma, follicular thyroid cancer, thymus carcinoma, and lung cancer. Different tumor types may affect the prognosis of patients with spinal metastases [31,33].Tuberculosis (TB) and metastatic lesions in the spine (ML) are frequently diagnosed lesions in the vertebral column. The incidence of TB lesions in the spine ranks first among tuberculosis of bones and joints, accounting for approximately 50% of all skeletal TB cases [1]. And also, the spine is the most common site for metastasis of the cancer process, with about 60% of bone metastases occurring in metastatic lesions of the spine [2]. Although spinal tuberculosis and metastatic lesion to the spine are two different diseases, and both lesions showed vertebral body destruction and localized mass on imaging examination [3]. However, nonspecific back pain represents the most common symptom in both diseases. In later stages, both lesions have the potential to cause spinal cord compression, leading to neurologic sequelae including paraplegia, paraparesis, pelvic organ dysfunction in the form of partial or complete urinary retention, and GI disturbances [1,4]. Given the similar manifestations, differential diagnosis of the two diseases is a clinical challenge [3,5,6].Although histologic examination is the gold standard for the differential diagnosis of spinal tuberculosis and metastatic lesions, sometimes patients with acute symptomatic spinal cord compression require urgent surgery in the form of decompression of the spinal cord and its roots when there is no time to perform a prior biopsy. Surgeons usually make a differential diagnosis based on one or some specific features of the two diseases, such as the presence or absence of intervertebral space destruction, based on MRI and CT studies, erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) level(s), rather than a systematic scoring system. [5] Although these features are effective in most cases, it would be very difficult to distinguish atypical cases, such as a polylocal tuberculous spinal lesion without intervertebral lesion.Therefore, a systematic guide to the differential diagnosis of tuberculous (TP) vertebral lesions and metastatic spinal lesions (ML) is of great importance for physicians in phthisioorthopedics, oncology, and neurosurgery.Hence, it is important to develop a new method to improve the accuracy of recognizing spinal metastases from spinal tuberculosis in the outpatient department to help outpatients receive optimal therapy.In this study, we retrospectively analyzed the clinical characteristics of spinal metastases and spinal tuberculosis and confirmed five characteristics that can be obtained by an outpatient orthopedist as significant predictors of spinal metastases, and developed an outpatient evaluation system. We also we validated the effectiveness of this scoring system and confirmed that it could improve the ability to distinguish spinal metastases from spinal tuberculosis.As advances in cancer treatment have improved patient survival, the prevalence of spinal metastases will inevitably increase [9,11,13].In this study, clinical, laboratory, and radiological data from 147 patients with tuberculous spondylitis and metastatic spinal lesions were systematically analyzed to develop and validate a new and practical scoring system for differential diagnosis between tuberculous and metastatic spinal column lesions.Patient selectionThis retrospective study included 147 patients with tuberculous spondylitis (n=77) and metastatic vertebral lesions (n=70) who were hospitalized in the Department of Bone and Joint Pathology at the Republican Specialized Scientific Research Center of Phthisiology and Pulmonology, named after Sh. A. Alimov, from 2020 to 2024.Patient selection was limited to meet the following inclusive criteria: (1) a definitive diagnosis of spinal tuberculosis or ML was confirmed histologically and immunohistochemically; and (2) patients who developed symptomatic compression of the spinal cord and its roots and required surgical intervention for abscessonecrectomy, sparing resection, and decompression of the spinal cord and its roots. In addition, patients with non-specific spinal lesions or primary tumor were excluded from this study. Data collectionClinical data included sex, age, history of tumor involvement of any organ, presence or absence of low-grade fever, night sweats and pain increasing at night. Laboratory data included white blood cell count (WBC), COE, CRP levels and tumor markers (AFP, PSA, CEA, CA199, CA125, CA15-3, CEA72-4). All the above findings were compared according to their normal ranges and values. X-ray radiologic findings (X-ray, CT and MRI) included omission lesions, destruction of the intervertebral space, compression pathologic fractures of vertebral bodies, para- and prevertebral and psoas abscesses, and compression of the spinal cord and its roots.Radiation study of the affected vertebrae showed that in patients with TS, the lesion of the 1st vertebra was observed in 5 patients (6.5%), whereas in the group with MSL, the lesion of the 1st vertebra was observed in 44 patients (62.8%); the most frequent lesion was 2 vertebrae, which was observed in 32 (41.5%) cases in patients with TS and in 13 (18.6%) in patients with MSL. Lesion of 3 vertebrae, was observed in 17 (22.0%) patients with TS and in 10 (14.3%). Lesions of 4 vertebrae were most rarely seen, in 13 (16.9%) with TS, and in 3 (4.3%) patients with MSL. Lesions of 4 or more vertebrae were observed in only 10 (12.9%) patients with tuberculous spondylitis (Table 1).
|
|
|
2. Results
- Patient CharacteristicsAs shown in Table 4, the total cohort consisted of 94 men and 53 women, with a mean age of 55.5 years (median 55; range 17-80).
|
|
|
3. Discussion
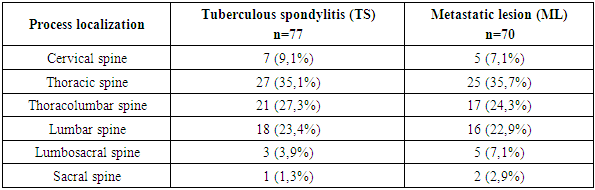
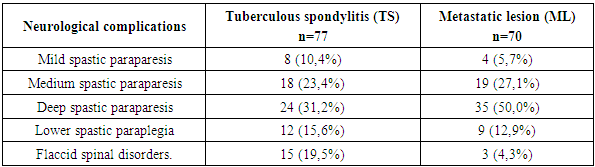
- Tuberculous spondylitis and metastatic lesions in the spinal cord (MSL) have similar clinical manifestations, which creates difficulties for clinicians in differential diagnosis, especially in some atypical cases. Although pathologic examination is the gold standard to confirm the diagnosis, performing biopsy in all patients with spinal cord compression is impractical because of the urgent need for surgical decompression. Ideally, it would be better if surgeons could quickly and accurately make a differential diagnosis with a convenient assessment tool. To address this problem, the present study developed a novel diagnostic scoring system to distinguish tuberculous spondylitis (TS) from metastatic spinal lesions (MSL) of ML. This scoring system consisted of 5 items determined by single-factor and multivariate analysis: increased pain at night (0 or 2 points), CRP levels (0 or 3 points), tumor marker values 0 or 2 points), minor lesions (0 or 3 points), and interbody destruction (0 or 3 points). Patients with a score greater than 7.5 points may be diagnosed with spinal tuberculosis, otherwise spinal ML. According to internal validation, the sensitivity and specificity of the system were 87.9% and 91.6%, respectively.Clinical factorsClinically, back pain is the most common symptom of spinal ML. Typically, pain is often increased at night due to distension of the epidural venous plexus and decreased secretion of endogenous corticoids [15]. In our study, we found that increased pain at night was an independent indicator of spinal ML. In spinal tuberculosis, back pain was also the most frequently reported symptom, followed by low-grade fever, weight loss, neurologic impairment, and night sweats [16].In our study, specific symptoms of tuberculosis such as low fever were considered and night sweats were selected for comparison. Although the results of single factor analysis in our study showed that slight fever of subfebrile nature and night sweating or morning sweating were factors for differential diagnosis, no significant difference was obtained in multivariate analysis. In addition, age and history of tumor development been found to be prognostic parameters that distinguish tuberculous pleural effusion from malignant pleural effusion, [17] and they are also widely used for differential diagnosis between spinal diseases. TB and ML in clinical practice, but our results showed that none of these parameters was considered as an independent diagnostic factor.Laboratory valuesCOE and CRP are usually elevated in most patients with active inflammatory tuberculosis. Although they are not unique to tuberculosis, elevation of COE and CRP is helpful in the diagnosis of tuberculous spondylitis. [18] Javed and coauthors suggested that COE is more sensitive in differentiating spinal tuberculosis from tumors than CRB, [19] while Sudprasert and coauthors suggested that CRB plays a more important role than COE in assessing the response to treatment and prognosis of spinal tuberculosis [20]. In our study, the results showed that CRP, rather than COE, was an independent diagnostic factor. Similarly, in patients with lymphocytic-exudative pleural effusion, elevated CRB level in pleural fluid was also an indicator to distinguish tuberculous pleurisy from malignant pleural effusion [21].Clinically, although tumor markers are mainly used to monitor cancer recurrence after treatment, only some of them are used for screening (e.g., AFP for hepatocellular carcinoma and PSA for prostate cancer). Elevated tumor marker concentration is an earlier sign of ML development than clinical manifestations and imaging studies [22]. In our study, due to the diversity of ML and limited sample size, a combination of tumor markers was used to screen for spinal ML. If any of the tumor marker factors were above normal, the patient would most likely be diagnosed with spinal ML.Radiologic factorsThe main role in the diagnosis of tuberculous spondylitis is played by X-ray tomographic methods, the sensitivity of which exceeds 80%, but the most specific visualization signs are considered to be contact destruction and pathological fractures on the background of abscesses, which is why common and complicated forms of TC reach 70% at the time of diagnosis.All the complexity of the structure and diversity of the clinical course of bone tumors cannot be understood based only on the ideas about the peculiarities of their cellular structure taken outside the whole sum of endogenous and exogenous factors. Hence the helplessness of purely morphological groupings and the uselessness for the clinician of endless morphological detailing of tumors based on their microscopic picture [51,53].Low specificity of patients' symptoms and radiologic signs can delay correct diagnosis leading to late administration of adequate therapy. This can often significantly affect the outcome in a negative way. Literature reports that this delay varies from 2 to 6 months or more [19] and various suggestions can be found in the scientific literature to help clinicians to make a clinical diagnosis. Therapy of spinal infection can be variable and multimodal, depending on the degree of infection, its localization in the spine, possible abscesses, etiology of infection and possible availability of equipment. A multidisciplinary approach to the clinical case is recommended with the participation of surgeons, vertebrologists, Consequently, patients with spinal pain, especially those in the above risk groups need early verification of the diagnosis. The leading role in the diagnosis of tuberculosis of the musculoskeletal system belongs to radiation methods of examination [39,41]. Depending on the duration and activity of the tuberculous process in the spine, the combined specificity of MRI and MSCT is quite high and exceeds 85% [32]. However, the longer and more active the course of tuberculous spondylitis, the more complications occur, reaching 80-100% especially in children. In the early stages, in the prespondylitic phase of tuberculous spondylitis, the combined specificity of MRI and MSCT is low. In addition, at the first symptoms of dorsopathy, only MRI with contrast can show only bone edema [39]. These factors lead to late diagnosis of bone and joint tuberculosis, including in our Republic, and disability due to this reaches 85%, and in 60% of cases disability occurs despite the implementation of a set of treatment measures [28].Identification of the causative agent is a key point in the management of inflammatory spinal diseases, which enables effective and targeted antimicrobial treatment [56].The literature suggests that involvement and erosion of vertebral closure plates, with possible disruption of the vertebral body architecture, is quite typical of infectious lesions of the vertebral bodies; the disease may spread to several segments. Abscesses, in some cases with calcification elements, and inflammatory exudate may be observed in the epidural space and/or para- and prevertebral soft tissues [23]. Posterior vertebral elements are usually preserved. On MRI images, a well-defined paraspinal area with abnormal signal intensity; a thin, smooth abscess wall; subligamentous spread to three or more vertebral levels; and multiple vertebral lesions are more suggestive of tuberculous spondylitis than purulent spondylitis. Bone fragments of intra- and/or extra-spinal soft tissues have been described as characteristic of spinal tuberculosis in combination with convex deformity and severe spinal collapse, even though these features are most obvious in advanced stages, when already the phthyseoorthopedist has to treat and even operate on the findings of the disease [49].Visualization tests can usually distinguish spinal tuberculosis from ML. Typical manifestations of spinal tuberculosis are destroyed adjacent vertebral bodies with involvement of intervertebral discs and paravertebral abscesses; whereas spinal tuberculosis mainly manifests as destruction of vertebral bone and soft tissue formation with rare disc involvement [3]. In addition to disc changes, a comparative study showed the presence of combined lesions of vertebral body and its posterior elements, skip lesions, solitary lesions, concentric collapsing abscess formation, etc. were MRI imaging of lower extremities methods of differentiating cases of spinal tuberculosis and ML were obtained. [5] In our study, spinal cord injury and intervertebral space destruction were defined as two independent diagnostic factors and included in the scoring system. As for other imaging features, take paraspinal abscess as an example, it is a manifestation that is highly suggestive of spinal tuberculosis, but sometimes cases of tuberculosis are in the developmental stage when the abscess has not yet formed (high specificity but low sensitivity).Despite the presence of a characteristic imaging finding, a misdiagnosis may still be made when spinal or MSL tuberculosis is suspected. For example, a case of atypical spinal TB with non-contiguous multiple bone destruction without paravertebral abscess or destruction of adjacent intervertebral discs [8], a case of isolated intraspinal TB without destruction of the vertebral body, vertebral arch, or intervertebral disc [9], and a case of TB with the presence of a solitary pulmonary nodule [10] have all been misdiagnosed as spinal ML. Similarly, due to radiologic similarities, it is also possible that patients with atypical spinal ML may also be misdiagnosed and treated for tuberculosis [12-14]. To better differentiate spinal tuberculosis from ML, advanced imaging techniques such as perfusion computed tomography and dynamic contrast MRI have been investigated and have shown promising results [3,23], but they have been limited for routine clinical use due to cost and time constraints. Moreover, the diagnosis of spinal tuberculosis or ML should not be based on imaging alone, and differentiation requires a combination of clinical, laboratory and radiological aspects. Thus, this scoring system may be effective. is a useful tool for differential diagnosis. It is comprehensive and practical, yet highly accurate.
4. Clinical Example
- Case 1
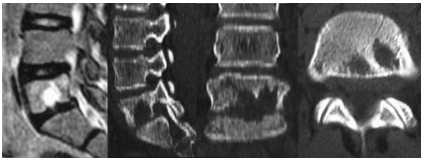 Patient is 35 years old. She had no pain that increased at night (2 points). Laboratory tests showed higher CRP (3 points) and cancer marker (AFP) (0 points). Radiologic examination showed focal spinal lesions (3 points), intervertebral space destruction (0 points). Her total score was 8 points and thus the presumptive diagnosis was spinal tuberculosis.According to complaints, moderate pain syndrome and subfebrile temperature for several months. The results of MRI performed at the beginning of the disease were considered as hemangioma; CT scan 2 months later revealed a contact destruction of L5-S1 with a deep focus in L5. Tuberculous spondylitis was histologically and bacteriologically verified.Case 2
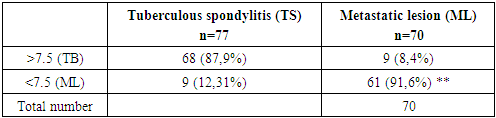
Patient is 35 years old. She had no pain that increased at night (2 points). Laboratory tests showed higher CRP (3 points) and cancer marker (AFP) (0 points). Radiologic examination showed focal spinal lesions (3 points), intervertebral space destruction (0 points). Her total score was 8 points and thus the presumptive diagnosis was spinal tuberculosis.According to complaints, moderate pain syndrome and subfebrile temperature for several months. The results of MRI performed at the beginning of the disease were considered as hemangioma; CT scan 2 months later revealed a contact destruction of L5-S1 with a deep focus in L5. Tuberculous spondylitis was histologically and bacteriologically verified.Case 2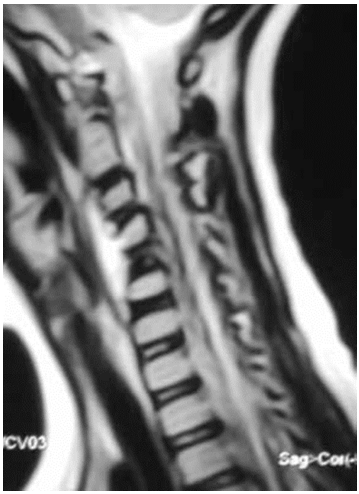 Patient is 66 years old. Patient had no pain that increased at night (2 points). Laboratory tests showed higher levels CRP (3 points) and normal tumor markers (2 points). Radiologic examination revealed minor lesions (0 points) without intervertebral space destruction (0 points). The total score was 7 points, and thus the presumptive diagnosis was spinal ML.At initial diagnosis, a homogeneous prevertebral component was considered a typical abscess from a destroyed C5 vertebra. He underwent surgery without a previous biopsy; granulation tissue was found in the abscess, but histologically, a histiocytic lesion was confirmed immunohistochemically. Radioisotope scanning revealed a second bone focus (destruction of the scapula), after which the patient was referred to an oncologic hospital for treatment.The total scores along with the predicted diagnoses of all patients in the validation cohort were calculated using the newly proposed scoring system as indicated above were examples were demonstrated. Comparison of predicted diagnoses with actual pathologic diagnoses showed that 68 out of 77 cases of tuberculosis and 61 out of 70 cases of ML were correctly predicted (Table 7).
Patient is 66 years old. Patient had no pain that increased at night (2 points). Laboratory tests showed higher levels CRP (3 points) and normal tumor markers (2 points). Radiologic examination revealed minor lesions (0 points) without intervertebral space destruction (0 points). The total score was 7 points, and thus the presumptive diagnosis was spinal ML.At initial diagnosis, a homogeneous prevertebral component was considered a typical abscess from a destroyed C5 vertebra. He underwent surgery without a previous biopsy; granulation tissue was found in the abscess, but histologically, a histiocytic lesion was confirmed immunohistochemically. Radioisotope scanning revealed a second bone focus (destruction of the scapula), after which the patient was referred to an oncologic hospital for treatment.The total scores along with the predicted diagnoses of all patients in the validation cohort were calculated using the newly proposed scoring system as indicated above were examples were demonstrated. Comparison of predicted diagnoses with actual pathologic diagnoses showed that 68 out of 77 cases of tuberculosis and 61 out of 70 cases of ML were correctly predicted (Table 7).
|
5. Conclusions
- Thus, the sensitivity and specificity of the new scoring system for differentiating spinal tuberculosis from ML were 87.9% and 91.6%, respectively.The present study has several limitations. It is a retrospective study and the cases are limited to one institution. The lack of external validation of the scoring system can be considered as a serious drawback. Second, to make the scoring system simple and practical, the laboratory data were dichotomized according to the normal range rather than the more optimal threshold determined by the ROC curve. In addition, the proposed diagnostic scoring system can be considered simply as an auxiliary tool, whereas histologic confirmation of the presumed diagnosis by biopsy is always the gold standard.Complicated forms of tuberculous spondylitis occur in 70% of adult patients with tuberculous spondylitis. Duration of the disease before diagnosis, and therefore disability ranges from 8 months to 1 year and more. In 60% of cases of paresis and paralysis, even a complex of therapeutic measures does not restore the patient's ability to work. In conclusion, by systematically analyzing clinical, laboratory, and radiologic factors, we determined that pain increased at night, CRP, oncomarker scores, lesion foci, and intervertebral space destruction were independent factors for differentiating spinal tuberculosis from ML. Moreover, a new diagnostic scoring system based on independent diagnostic factors and internal validation was developed. Further external validation using new samples and independent for this system to be accepted for general use, several evaluations are needed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML