-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(3): 496-500
doi:10.5923/j.ajmms.20251503.02
Received: Feb. 4, 2025; Accepted: Feb. 24, 2025; Published: Mar. 5, 2025

Assessment of the Condition of Newborns Delivered by Cesarean Section under Maternal Sedation with Various Medications
Khudoyberdiyeva Gulrukh, Matlubov Mansur, Khamdamova Eleonora
Department of Anesthesiology, Resuscitation and Emergency Midicine, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Khudoyberdiyeva Gulrukh, Department of Anesthesiology, Resuscitation and Emergency Midicine, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In obstetric anesthesiology, considering perinatological characteristics, approaches to the concept of optimal anesthesia differ significantly from traditional methods used in general anesthesiology. In this context, the primary objective is not only to protect the mother from the consequences of surgical intervention but also to minimize the impact of medications on the fetus. A key factor is preserving the newborn's ability to rapidly adapt after birth, which requires a specialized approach to the selection of anesthetic agents and the techniques of their administration.
Keywords: Cesarean section, Sedation, Pregnant women, Anesthesia, Newborns, Dexmedetomidine
Cite this paper: Khudoyberdiyeva Gulrukh, Matlubov Mansur, Khamdamova Eleonora, Assessment of the Condition of Newborns Delivered by Cesarean Section under Maternal Sedation with Various Medications, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 496-500. doi: 10.5923/j.ajmms.20251503.02.
Article Outline
1. Introduction
- Cesarean section is one of the most commonly performed surgical procedures worldwide, accounting for a significant proportion of births in both developed and developing countries. The choice of anesthesia and sedation during cesarean delivery plays a crucial role in ensuring maternal safety while minimizing potential adverse effects on the newborn. In obstetric anesthesiology, the concept of "optimal anesthesia" differs considerably from that in general anesthesiology due to the unique physiological changes of pregnancy and the dual focus on both maternal and fetal well-being [1-5].Spinal anesthesia is widely preferred for cesarean sections due to its rapid onset, effective analgesia, and favorable safety profile. However, managing maternal anxiety and stress during surgery often necessitates the use of sedative medications. The selection of sedative agents is critical, as many drugs can cross the placental barrier, potentially affecting fetal neurodevelopment and early postnatal adaptation [6].Dexmedetomidine, a selective α2-adrenergic receptor agonist, has gained attention for its sedative, anxiolytic, and analgesic properties with minimal respiratory depression. Its use in obstetric anesthesia is of growing interest due to potential benefits in maintaining neonatal adaptive capacity. Conversely, commonly used sedatives such as ketamine and propofol, while effective for maternal sedation, have been associated with varying degrees of neonatal depression when administered before delivery. The timing of sedative administration also plays a significant role, as administering drugs after umbilical cord clamping may reduce neonatal exposure and its associated risks [7-8].This study aims to evaluate the clinical and functional adaptation of newborns delivered via cesarean section under maternal sedation with various medications, focusing on the comparative effects of dexmedetomidine, ketamine, propofol, sibazone, and sodium oxybate. By analyzing neonatal outcomes through Apgar scores and the Neonatal Adaptive Capacity Score (NACS), this research seeks to provide evidence-based insights into the safest and most effective sedation practices in obstetric anesthesia.
2. Materials and Methods
- This study presents data obtained from a prospective analysis of medical records, anesthesia charts, intensive care monitoring sheets, and the results of functional and biochemical assessments of 200 newborns. These newborns were delivered by mothers without severe somatic pathologies, classified as ASA physical status II, with gestational ages ranging from 37 to 39 weeks. The deliveries took place in the Department of Obstetrics at the Multidisciplinary Clinic of Samarkand State Medical University [9-10].All newborns were divided into five groups:• Group 1 (Main Group): Included 100 newborns delivered under maternal sedation with dexmedetomidine.• Group 2 (Control Group): Included 25 newborns delivered under maternal sedation with ketamine.• Group 3 (Control Group): Included 25 newborns delivered under maternal sedation with propofol.• Group 4 (Control Group): Included 25 newborns delivered with maternal sedation using sibazone, administered after umbilical cord clamping.• Group 5 (Control Group): Included 25 newborns delivered with maternal sedation using sodium oxybate, administered after umbilical cord clamping.All cesarean sections were elective procedures. The maternal groups were comparable in baseline characteristics, with the only difference being the type of sedative medication administered during spinal anesthesia.The study included 100 newborns in the dexmedetomidine group and 25 in each control group. This distribution was based on a power analysis, which determined that a larger sample size in the primary study group was necessary to detect significant differences in neonatal outcomes with adequate statistical power (80%, α = 0.05). The power analysis was performed using neonatal Apgar scores as the primary outcome measure, with a minimum sample of 100 in the dexmedetomidine group to ensure sufficient effect size detection. The control groups were designed with a smaller sample size due to resource limitations and ethical considerations regarding sedation with drugs known to have potential neonatal depressive effects.All numerical data obtained from the study were processed using variational statistical methods, with Student’s t-test applied for statistical analysis.The indications for spinal anesthesia included uterine scars from previous cesarean sections, a history of conservative myomectomy, pregnancy complicated by uterine fibroids or other gynecological pathologies, as well as placenta previa.All women underwent compression bandaging of the lower extremities in the operating room, followed by intravenous premedication with diphenhydramine (0.2 mg/kg) and dexamethasone (0.07 mg/kg). After preventive infusion of saline solutions (6–8 mL/kg), spinal anesthesia was performed in the sitting position under local infiltration anesthesia. A 25G “Pencil Point” needle was used to puncture the subarachnoid space at the L₂–L₄ level. As local anesthetics, either a 0.5% isobaric solution of bupivacaine (HWARDS, Pakistan) or a 0.5% hyperbaric solution of Longocaine-Heavy (“Yuria-Pharm”) was administered in a dose of 12 ± 3.0 mg [1-13].To prevent aortocaval compression before fetal extraction, patients were positioned in a 20° left uterine displacement. The intraoperative fluid management protocol primarily involved the infusion of crystalloids and balanced colloid solutions at a rate of 8–10 mL/kg/hour. Surgery commenced 10–15 minutes after achieving clinical signs of an adequate segmental sensory-motor block at the level required for the procedure.
2.1. Sedation Protocols
- To reduce psycho-emotional stress, the following sedative regimens were used:Group 1 (Main Group): Dexmedetomidine was administered with a loading dose of 1 mcg/kg over 10 minutes, followed by a maintenance dose of 0.2–0.7 mcg/kg/hour throughout the surgery until completion.Control Groups (2–5): Pregnant women were divided into subgroups of 25 patients each, depending on the sedative agent used:Group 2: Ketamine at 0.5–0.7 mg/kg as the hypnotic component.Group 3: Propofol at 5–10 mcg/kg/min as the hypnotic component.Group 4: Sibazone at 0.2 mg/kg, administered after umbilical cord clamping.Group 5: Sodium oxybate at 20–40 mg/kg, administered after umbilical cord clamping.It is important to note that in Groups 4 and 5, sedation was initiated only after the umbilical cord was clamped to minimize potential drug exposure to the fetus.
2.2. Postoperative Management and Neonatal Assessment
- All operated patients were transferred to the intensive care unit, where syndrome-based therapy was provided, along with continuous monitoring of vital systems. The condition of newborns was assessed using the Apgar score at the 1st and 5th minutes of life. Additionally, the Neonatal Adaptive Capacity Score (NACS) was used to evaluate newborns at 1 hour and 24 hours after birth.Neonatal adaptation was assessed using standardized intervention criteria, ensuring that clinical decisions regarding neonatal care followed internationally accepted guidelines. Newborns received interventions based on the following criteria:• Oxygen therapy: Initiated for SpO₂ < 90% at 1 minute or persistent cyanosis.• Neonatal Intensive Care Unit (NICU) admission: Required for severe respiratory distress, persistent hypotonia, or Apgar score ≤6 at 5 minutes.• Airway suctioning: Performed if significant meconium-stained amniotic fluid was noted.• Positive pressure ventilation (PPV): Administered if the newborn exhibited absent or inadequate spontaneous breathing.All neonatal interventions followed the American Academy of Pediatrics (AAP) Neonatal Resuscitation Program (NRP) guidelines, ensuring uniform resuscitation standards across all groups.
2.3. Potential Bias and Confounders
- To minimize confounding factors, we considered maternal demographic and clinical characteristics, including:Age, BMI, parity, and pre-existing comorbidities (e.g., gestational diabetes, hypertension), ensuring balanced distribution across study groups. No significant differences were found among groups in these variables (p > 0.05, ANOVA test).Exclusion criteria: Newborns requiring intubation, CPR, or major resuscitative interventions beyond standard neonatal care were excluded to maintain homogeneity in neonatal outcomes.Statistical adjustment: To control for potential residual confounders, multivariate regression analysis was performed, adjusting for maternal age, BMI, and comorbidities to ensure the validity of neonatal outcome comparisons.
2.4. Statistical Analysis
- All numerical data were processed using variational statistical methods. To compare the differences among groups:• Student’s t-test was used for pairwise comparisons of normally distributed continuous variables (e.g., Apgar scores, Neonatal Adaptive Capacity Score [NACS]).• One-way ANOVA was performed for comparisons across multiple groups, followed by Bonferroni post-hoc tests to account for multiple comparisons and reduce the risk of Type I errors.• Categorical variables were analyzed using chi-square tests or Fisher’s exact test, where appropriate.• Data were expressed as mean ± standard deviation (SD), with p < 0.05 considered statistically significant.All statistical analyses were conducted using SPSS v.26 (IBM Corp., USA).
3. Results
- All newborns were full-term, and their birth weights did not show any statistically significant differences across the study groups. The evaluation of newborns using the Apgar score revealed a significant decrease in scores in Groups 2 and 3 compared to Group 1 (p < 0.001).• At the first minute of life, newborns in Group 1 (dexmedetomidine) had an average Apgar score of 8.1 ± 0.1, while those in Group 2 (ketamine) and Group 3 (propofol) had significantly lower scores of 7.6 ± 0.1 and 7.1 ± 0.1, respectively.• By the fifth minute, the Apgar score improved in all groups; however, newborns in Group 1 reached 9.3 ± 0.2, whereas those in Groups 2 and 3 showed lower scores of 8.4 ± 0.2 and 8.3 ± 0.1, respectively (p < 0.001).The clinical adaptation patterns of newborns delivered via cesarean section under maternal sedation with ketamine and propofol (Groups 2 and 3) demonstrated more frequent occurrences of the following complications compared to Group 1:• Impaired establishment of spontaneous breathing• Pronounced muscle hypotonia• Increased need for urgent upper airway suctioning• Requirement for mask oxygenationThese findings suggest that dexmedetomidine provides better neonatal outcomes in terms of immediate postnatal adaptation compared to ketamine and propofol when used for maternal sedation during cesarean section under spinal anesthesia.
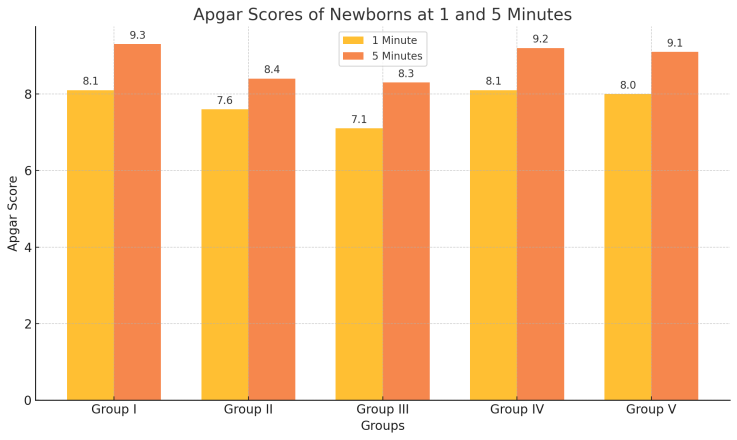 | Figure 1. Apgar Scores of Newborns at 1 and 5 minutes |
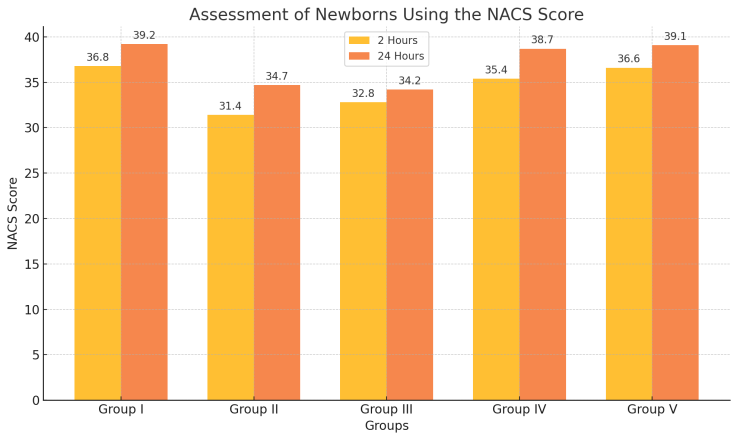 | Figure 2. Assessment of Newborns Using the NACS Score |
3.1. Neuropsychological Adapatation Assessment
- The evaluation of neuropsychological adaptation using the Neonatal Adaptive Capacity Score (NACS), specifically in tests assessing passive tone, active tone, and physiological reflexes, revealed significantly higher scores in newborns from Group 1 (dexmedetomidine) and from Groups 4 and 5 (where sedatives were administered after umbilical cord clamping) compared to those in Groups 2 (ketamine) and 3 (propofol).• At 2 hours after birth, newborns in Groups 1, 4, and 5 demonstrated superior adaptive abilities, with better muscle tone and more robust physiological reflexes.• At 24 hours after birth, a similar trend persisted, confirming sustained neuropsychological stability in these groups.These comparative findings from both the Apgar and NACS assessments clearly indicate that maternal sedation with dexmedetomidine during cesarean section under spinal anesthesia offers a distinct advantage in terms of the absence of depressive effects on the newborn.In contrast, ketamine and propofol used for maternal sedation exerted significant depressive effects on neonatal adaptation, as evidenced by lower scores in both early and late postnatal evaluations. This highlights the importance of selecting sedative agents that minimize neonatal risks during obstetric anesthesia.
4. Conclusions
4.1. Clinical Implications
- The findings of this study have direct clinical implications for obstetric anesthesiology: Anesthesiologists should consider dexmedetomidine as the preferred sedative agent for cesarean sections under spinal anesthesia due to its minimal impact on neonatal adaptation. The higher Apgar and NACS scores in this group suggest superior neonatal outcomes compared to ketamine and propofol. Timing of sedation is critical. Groups receiving sibazone and sodium oxybate only after umbilical cord clamping demonstrated better neonatal adaptation than those exposed to sedation before delivery. This reinforces the practice of delaying maternal sedation until after fetal extraction to avoid neonatal depressive effects. Personalized sedation strategies should be developed, considering maternal comorbidities and fetal well-being, rather than a one-size-fits-all approach.These findings can inform clinical guidelines and hospital protocols for safer obstetric anesthesia practices.
4.2. Study Limitations
- While this study provides valuable insights into neonatal outcomes under different sedation protocols, several limitations must be acknowledged:Single-center design – The study was conducted at one institution, limiting the generalizability of the findings to other settings with different patient populations, anesthesia protocols, or surgical conditions.Small sample size for control groups – While the primary group had adequate statistical power, the control groups had smaller sample sizes, which may impact the robustness of subgroup comparisons.Lack of long-term follow-up – This study focused on immediate neonatal adaptation (Apgar and NACS scores), but did not assess long-term neurodevelopmental outcomes. Future research should evaluate cognitive and motor development over months or years.Selection bias – Only ASA II patients were included to maintain sample homogeneity, which excludes high-risk pregnancies. The results may not apply to more complex obstetric cases.Observer bias – Apgar scores were assigned by the attending neonatal team. While they followed standardized guidelines, inter-observer variability remains a potential limitation. Future studies should consider blinded assessments or automated scoring tools to minimize bias.
4.3. Future Research Directions
- While this studsy provides important insights into the effects of maternal sedation on neonatal adaptation, further research is necessary to expand our understanding and improve obstetric anesthesia practices. Future studies should focus on:1. Comparing dexmedetomidine with newer sedative agents – As anesthetic and sedative pharmacology continues to evolve, newer drugs with potentially superior safety profiles should be investigated. Comparative studies assessing the neonatal and maternal effects of remimazolam, remifentanil, or novel α₂-adrenergic agonists could provide valuable clinical insights.2. Long-term neurodevelopmental outcomes of newborns exposed to maternal sedation – While this study assessed immediate postnatal adaptation, prospective cohort studies following newborns over months or years are needed to evaluate whether intrauterine exposure to different sedatives has lasting effects on cognitive function, motor skills, and neurodevelopment.3. Investigating combined sedation techniques – Future research should explore whether combining low-dose dexmedetomidine with other agents (e.g., fentanyl, midazolam, or propofol) could optimize maternal comfort while minimizing neonatal depressive effects. Studying multimodal sedation protocols that balance efficacy and safety is critical for refining anesthesia practices.4. Individualized sedation protocols based on maternal and fetal factors – Given the variability in maternal physiology and fetal response to anesthesia, personalized sedation strategies considering maternal BMI, placental function, and fetal maturity could enhance neonatal outcomes. Machine learning models predicting optimal sedative doses based on patient characteristics could be an area of future exploration.5. Multicenter studies for external validation – Conducting large-scale, multicenter randomized controlled trials (RCTs) would improve the generalizability of findings, ensuring that results apply across different healthcare settings and diverse populations.By addressing these areas, future research can further optimize sedation practices for cesarean sections, ultimately improving both maternal safety and neonatal health.The comparative assessment of the Apgar and NACS scores clearly demonstrates that maternal sedation with dexmedetomidine during cesarean section under spinal anesthesia offers a distinct advantage due to the absence of depressive effects on the newborn. This approach effectively preserves the adaptive and compensatory capabilities of the newborn’s body during the early postnatal period, facilitating a smoother transition to extrauterine life.
ACKNOWLEDGEMENTS
- We would like to express our deepest gratitude to the Department of Anesthesiology, Resuscitation, and Emergency Medicine at Samarkand State Medical University for their invaluable support and guidance throughout this research. Special thanks to all the medical staff and patients who participated in the study, whose cooperation and dedication made this work possible.We are profoundly thankful to assistent-professor Nematulloev Tukhtasin Komiljonovich for his expert supervision, insightful feedback, and constant encouragement, which greatly contributed to the successful completion of this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML