Abduolimov A. A.1, Mamajanov B. S.2
1Fergana Public Health Medical Institute, Uzbekistan
2Andijan State Medical Institute, Uzbekistan
Correspondence to: Abduolimov A. A., Fergana Public Health Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Hypertension-induced atherosclerosis leads to stenosis of medium and small-caliber blood vessels in the brain, ultimately resulting in ischemic necrosis in the affected area. In particular, atherosclerosis caused by hypertension manifests as vascular deformations such as aneurysms and stenosis. In many cases, hypertension-associated atherosclerosis presents with bilateral metabolic disturbances and stromal vascular dystrophy, leading to characteristic cerebrovascular damage. The development of atheromatous changes in the endothelial and subendothelial layers of blood vessels results in luminal narrowing, which contributes to ischemic processes.
Keywords:
Cerebrovascular disease, Atherosclerosis, Atheromatosis, Morphology, Hypertension
Cite this paper: Abduolimov A. A., Mamajanov B. S., Hypertension as a Complication in Cerebrovascular Diseases: Morphological Changes in Intracerebral Blood Vessels, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 470-472. doi: 10.5923/j.ajmms.20251502.43.
1. Introduction
Research based on global population studies indicates that, over the past forty years, cerebral ischemia and encephalomalacia have been most frequently observed in individuals aged 49 to 55 [1,2,4]. Considering that 70-75% of cerebrovascular pathologies are influenced by hypertension and obesity, the incidence of atherosclerosis in the arteries forming the Circle of Willis has been linked to various neurological and cognitive impairments [3,12]. Studies suggest that 40-56% of cerebrovascular diseases are associated with atherosclerosis in the Circle of Willis [5,6,7,8].The localization of atherosclerotic lesions contributes to varying degrees of vascular damage: the anterior communicating and anterior cerebral arteries are affected in 40-50% of cases, the internal carotid and posterior communicating arteries in 15-20%, the middle cerebral artery in 15-20%, the basilar and posterior cerebral arteries in 5%, and other arteries in 4-9% of cases [9,10,11,13,14].
2. Discussion and Results
In patients with hypertension, progressive arterial narrowing, exacerbated by chronic hemodynamic disorders in the Circle of Willis, is commonly observed after the age of 50-55, particularly under stress-related conditions. The narrowing of blood vessels leads to chronic hemodynamic disturbances, resulting in lipid plaque accumulation in the endothelial layer. As this process intensifies, atheromatous changes in the subendothelial layer lead to intimal damage, which in turn facilitates the formation of atherosclerotic plaques and further vascular lumen reduction.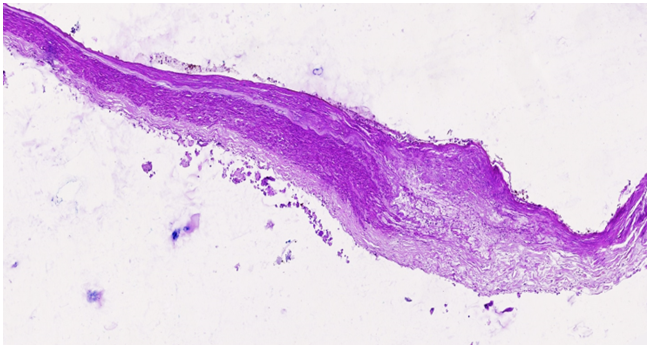 | Figure 1. The right cerebral artery wall shows an atherosclerotic plaque with an underlying atheromatous mass. The endothelial surface of the plaque varies in appearance, acting as a primary factor in thrombogenesis. Sclerotic changes are evident in the muscle layer. Staining: H.E. Magnification: 4x10 |
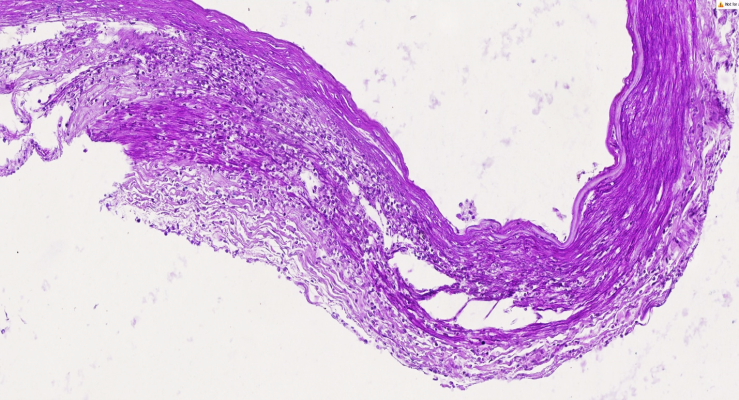 | Figure 2. The right cerebral artery exhibits an atherosclerotic plaque with myolysis and macrophage infiltration in the medial layer. The endothelial surface of the plaque presents diverse structural variations, contributing to thrombogenesis. The muscle layer has developed sclerotic changes. Staining: H.E. Magnification: 10x10 |
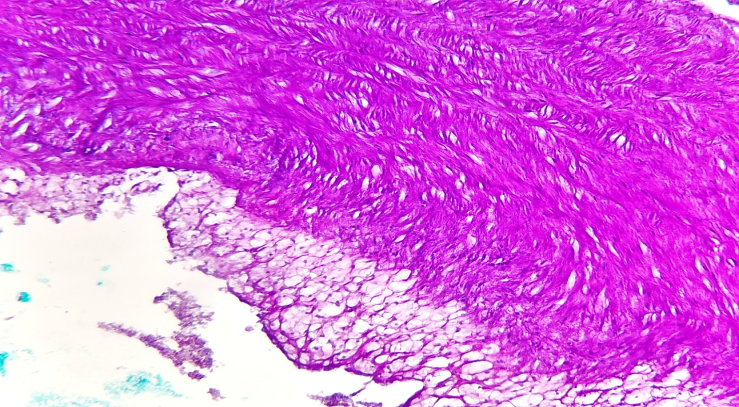 | Figure 3. The right cerebral artery displays an atherosclerotic plaque with a lipid deposit. The medial layer shows sclerotic changes with disorganized coarse fibrous structures. The endothelial surface of the plaque varies, serving as a primary factor in thrombogenesis. The muscle layer exhibits advanced sclerosis. Staining: H.E. Magnification: 40x10 |
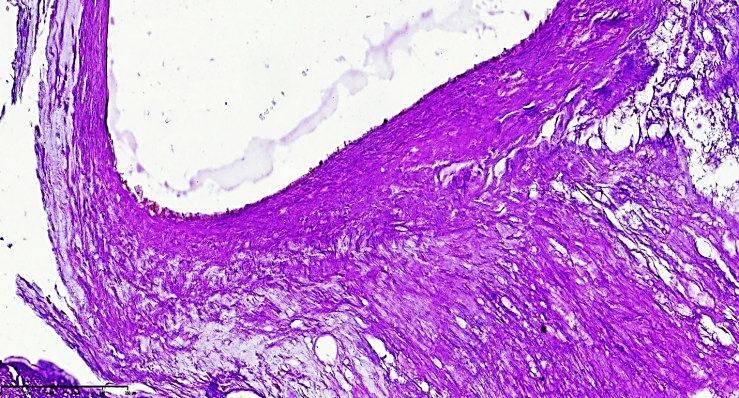 | Figure 4. Post-atherosclerotic myofibrosis and atheromatous foci are observed in the right cerebral artery. The vascular histoarchitecture is significantly altered, consisting mainly of coarse fibrous connective tissue due to sclerosis. The endothelial surface of the plaque remains irregular, contributing to thrombogenesis. Staining: H.E. Magnification: 40x10 |
The most interesting aspect is that the diameter of the anterior cerebral artery is small (3-5 mm), and when the pulse pressure increases, oscillations occur in the vascular wall, and the contraction of the muscular layer of this artery leads to mechanical friction of the intima and chronic damage to the endothelial layer due to an increase in surface elastic resistance. As a result, the permeability of the vascular wall increases, which gradually leads to plasmatic thickening of the vascular wall, mucoid thickening. Of course, in this case, the turbulent flow in the narrowed arterial lumen leads to the accumulation of various types of complex lipids of different densities in the plasma precisely on the surface of the anterior cerebral artery wall. As a result, the intimal area where the thickening occurs affects the narrowing of the vascular wall and the intensity of blood flow, leading to the development of mucoid thickening, fibrinoid thickening and arteriosclerosis in the vascular wall. It leads to the development of metabolic arteriosclerosis in the vessel.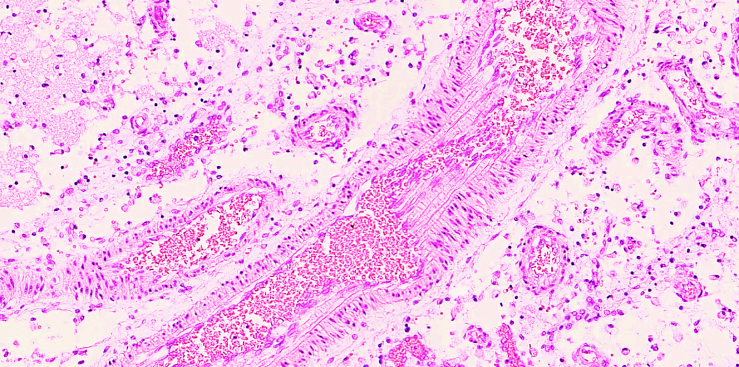 | Figure 5. Secondary branch of the right cerebral artery displaying an atherosclerotic plaque and varying wall thickness. A stenotic focus is identified. Staining: H&E, Magnification: 20×10 |
Progressive destruction and fragmentation of elastic and collagen fibers impair the functional integrity of the muscular layer, leading to fusiform aneurysms. In certain conditions, such as pregnancy or toxicosis, endothelial-damaging factors may contribute to saccular aneurysms in the posterior communicating and middle cerebral artery territories, though the exact pathogenic mechanisms remain unclear.
3. Conclusions
One of the primary vascular changes in hypertension is the development of atherosclerosis, leading to narrowing of the cerebral arteries. Hypertension induces dystrophic and sclerotic changes in the vessel walls, resulting in sequential structural damage and the formation of atheromatous plaques. The destruction of collagen and elastic fibers leads to atherosclerosis, characterized by elastolysis, collagenolysis, and fibromuscular dysplasia. The Circle of Willis, particularly the anterior communicating artery, is most affected, undergoing significant structural remodeling characteristic of atherosclerotic pathology.
References
| [1] | Kim DK, Lee HS, Park JY, Kim JW, Ha JS, Kim JH, Yang WJ, Cho KS. Does androgen-deprivation therapy increase the risk of ischemic cardiovascular and cerebrovascular diseases in patients with prostate cancer? A nationwide population-based cohort study. // J Cancer Res Clin Oncol. 2021 Apr; 147(4): 1217-1226. |
| [2] | Kim J, Freeman K, Ayala A, Mullen M, Sun Z, Rhee JW. Cardiovascular Impact of Androgen Deprivation Therapy: from Basic Biology to Clinical Practice. // Curr Oncol Rep. 2023 Sep; 25(9): 965-977. |
| [3] | Nakanishi K, Jin Z, Homma S, Elkind MSV, Rundek T, Tugcu A, Sacco RL, Di Tullio MR. Association of Blood Pressure Control Level With Left Ventricular Morphology and Function and With Subclinical Cerebrovascular Disease. // J Am Heart Assoc. 2017 Jul 30; 6(8): e006246. |
| [4] | Salvetti M, Paini A, Bertacchini F, Stassaldi D, Aggiusti C, Agabiti Rosei C, Bassetti D, Agabiti-Rosei E, Muiesan ML. Changes in left ventricular geometry during antihypertensive treatment. // Pharmacol Res. 2018 Aug; 134: 193-199. |
| [5] | Yamamoto N, Sato K, Tokitsu T, Taguchi T, Shiosakai K, Sugimoto K, Tsujita K; ESES-LVH investigators. Efficacy and Safety of Esaxerenone in Hypertensive Patients with Left Ventricular Hypertrophy (ESES-LVH) Study: A Multicenter, Open-Label, Prospective, Interventional Study. Adv Ther. 2024 Mar; 41(3): 1284-1303. |
| [6] | Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology. // J Hypertens. 2018; 36: 2284–2309. |
| [7] | Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. // J Am Coll Cardiol. 2018; 71: e127–248. |
| [8] | Levy PD, Burla MJ, Twiner MJ, Marinica AL, Mahn JJ, Reed B, Brody A, Ehrman R, Brodsky A, Zhang Y, Nasser SA, Flack JM. Effect of Lower Blood Pressure Goals on Left Ventricular Structure and Function in Patients With Subclinical Hypertensive Heart Disease. //Am J Hypertens. 2020 Sep 10; 33(9): 837-845. |
| [9] | Tanaka M. Functional Vascular Anatomy of the Brain. // Neurol Med Chir (Tokyo). 2017 Nov 15; 57(11): 584-589. |
| [10] | Wan Z, Meng H, Xu N, Liu T, Chen Z, Zhang Z, Xu J, Wang H. Coil embolisation of multiple cerebral aneurysms with lateral type I persistent primitive trigeminal artery: A case report and literature review. // Interv Neuroradiol. 2019 Dec; 25(6): 628-634. |
| [11] | Heiss WD. PET imaging in ischemic cerebrovascular disease: current status and future directions. Neurosci Bull. 2014 Oct; 30(5): 713-32. |
| [12] | Wang Z, Mascarenhas C, Jia X. Positron Emission Tomography After Ischemic Brain Injury: Current Challenges and Future Developments. // Transl Stroke Res. 2020 Aug; 11(4): 628-642. |
| [13] | Baskin A, Buchegger F, Seimbille Y, Ratib O, Garibotto V. PET Molecular Imaging of Hypoxia in Ischemic Stroke: An Update. // Curr Vasc Pharmacol. 2015; 13(2): 209-17. |
| [14] | Zhang L, Li Y, Bian L, Luo Q, Zhang X, Zhao B. Cognitive Impairment of Patient With Neurological Cerebrovascular Disease Using the Artificial Intelligence Technology Guided by MRI. // Front Public Health. 2022 Mar 3; 9: 813641. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


