-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(2): 466-469
doi:10.5923/j.ajmms.20251502.42
Received: Jan. 26, 2025; Accepted: Feb. 20, 2025; Published: Feb. 28, 2025

A New Approach to Immunohistochemical and Morphofunctional Changes in the Liver as a Result of Groundwater Consumption
Azimov Oybek Olimovich, Esanova Nasiba Tukhsanovna
Bukhara State Medical Institute named after Abu Ali ibn Sino, Bukhara, Uzbekistan
Correspondence to: Esanova Nasiba Tukhsanovna, Bukhara State Medical Institute named after Abu Ali ibn Sino, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We studied the morphological and immunohistochemical characteristics of the liver as a result of groundwater consumption in an experimental study in rats. We came to the conclusion that the rat liver has a clearly expressed multi-lobed structure, contains classic liver lobules with a developed blood supply and bile secretion system. In an immunohistochemical study, the expression of Ki-67 (proliferative index) and Bcl-2 was estimated as a percentage. Quantitative assessment was carried out based on the relative percentage of stained cells. Immunohistochemical analysis revealed changes in the expression of apoptosis and inflammation markers, indicating possible adaptive-compensatory mechanisms.
Keywords: Hard water, Beneficial and harmful properties, Liver, Morphology, Immunohistochemical study
Cite this paper: Azimov Oybek Olimovich, Esanova Nasiba Tukhsanovna, A New Approach to Immunohistochemical and Morphofunctional Changes in the Liver as a Result of Groundwater Consumption, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 466-469. doi: 10.5923/j.ajmms.20251502.42.
1. Introduction
- Hard water is widespread in various regions, especially in places with high calcium and magnesium content in tap water. Its use over a long period of time can have a negative effect on the body. It is not surprising that newborns who spend the first three months of life in regions with hard water, according to a study in the Journal of Allergy and Clinical Immunology, have an 87% increased risk of eczema. Previously, similar studies were conducted on children from the USA, Spain and Japan. However, according to the same WHO document, water softeners have little effect on the situation, so it is probably not only the water that is at fault, but also the predisposition of the patients themselves. Mutations in the gene of the filaggrin protein, responsible for the protective barrier functions of the skin, slightly increase this risk [1,4,5]. At the same time, hard water can harm the body's systems with prolonged use, as it can harm the function of many internal organs, and at that time many ways to "soften" water were invented. Temporary hardness can be removed by simply boiling the water: hydrocarbonates will become scale after boiling. It is more difficult to combat permanent hardness; chemical methods are often used for this [3,6,8]. Drinking water hardness reduces the risk of hypertension and mortality from cardiovascular diseases. For example, according to a 1981 article, in cities in Great Britain with soft drinking water, mortality from cardiovascular diseases is 10-15% higher than in cities with medium-hard water (about 170 mg/l). Further increases in concentration do not significantly affect the statistics. A similar effect is described in a 2003 article by French scientists, in a study by scientists from Sweden, and other works. As the authors of the same WHO document note, some clinical trials show that taking large amounts of calcium supplements, on the contrary, increases the risk of kidney stones [7,12,13]. Perhaps it all depends on the form in which a person takes it: with supplements or with water and food. Hypercalcemia, i.e. excess calcium in the body, occurs much less frequently than a deficiency of this element. The fact is that excess calcium in healthy people is excreted through the kidneys, and only impaired renal function can put a person's health at risk. The story with magnesium is similar: it is unlikely that an excess of this element would harm a person with healthy kidneys. Only occasionally does consuming more than 250 mg of magnesium per day (for this amount you would have to drink five to ten liters of very hard water) produce a laxative effect on a healthy person, but the body adapts to it after some time. Statisticians often come to the conclusion that the higher the water hardness, the lower the mortality from cardiovascular diseases, but experiments do not confirm this relationship [10]. It is not entirely clear how to interpret the discrepancies. Perhaps some of the opposite results can be explained by the fact that those scientists who studied a large number of people usually do not take into account how much calcium and magnesium the participants in their studies could have received in other ways, not from water. Therefore, it is not yet possible to select the dosage of ions in drinking water for “useful” hardness, especially since different people need different amounts of calcium and magnesium depending on their gender, age and body weight. The positive effects of drinking hard water are not sufficiently confirmed [14]. But washing with hard water can cause peeling skin and sometimes even atopic dermatitis. One possible explanation is that we use more soap in hard water, since it foams worse. Not only does soap itself dry out the skin, but hard water, when interacting with it, forms salts that are difficult to wash off and can cause irritation in people with sensitive skin. Studies show that drinking hard water may be associated with the development of pathological processes in parenchymatous organs. Despite the availability of data on the effect of hard water on the cardiovascular system and kidneys, morphological changes in the liver with long-term use of such water have not been sufficiently studied. Purpose of the study. Development of new approaches to the study of morphofunctional, immunohistochemical changes in the liver when using groundwater with a high chemical composition, as well as methods of treatment and prevention.
2. Materials and Methods
- For the study, sexually mature outbred white rats aged 3–6 months weighing 160–180 g were selected and divided into the following groups: 1st control group – white rats aged 3–6 months receiving centralized drinking water; 2nd group – white rats aged 3–6 months receiving underground water with high chemical content; 3rd group – white rats aged 3–6 months receiving underground water with high chemical content, corrected with citric acid and a biologically active additive from the fruits of the medicinal asparagus plant; 4th group – white rats aged 3–6 months receiving underground water from the health resort “Jo‘yzar”. An in-depth study of morphological changes in the liver when consuming hard water will help identify potential risks and suggest preventive measures for the population living in regions with increased water hardness.
3. Results and Discussion
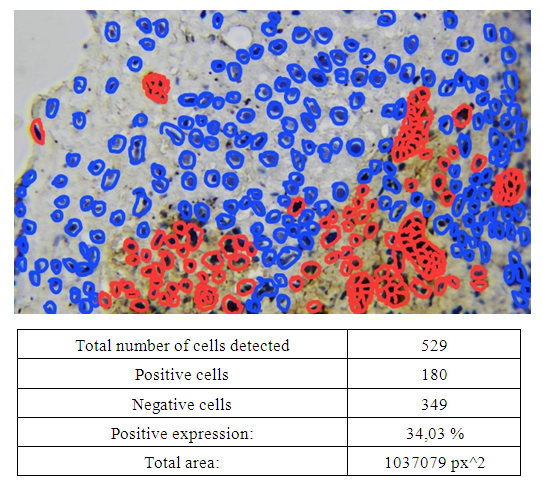
- For the study, sexually mature outbred white rats aged 3–6 months weighing 160–180 g were selected and divided into the following groups: 1st control group – white rats aged 3–6 months receiving centralized drinking water; 2nd group – white rats aged 3–6 months receiving underground water with high chemical content; 3rd group – white rats aged 3–6 months receiving underground water with high chemical content, corrected with citric acid and a biologically active additive from the fruits of the medicinal asparagus plant; 4th group – white rats aged 3–6 months receiving underground water from the health resort “Jo‘yzar”. An in-depth study of morphological changes in the liver when consuming hard water will help identify potential risks and suggest preventive measures for the population living in regions with increased water hardness. Morphological parameters of the liver were assessed in order to determine possible compensatory and adaptive reactions. The features of the cellular organization of the liver were studied using immunohistochemical markers (CD-45, Bcl-2) to identify signs of apoptosis and inflammatory reaction. The obtained data were compared with the control group consuming standard drinking water, and the extent of the detected changes was determined. Possible correlations were identified between the composition of groundwater and changes in hepatocytes and vascular structures of the liver (Fig. 1 and 2).
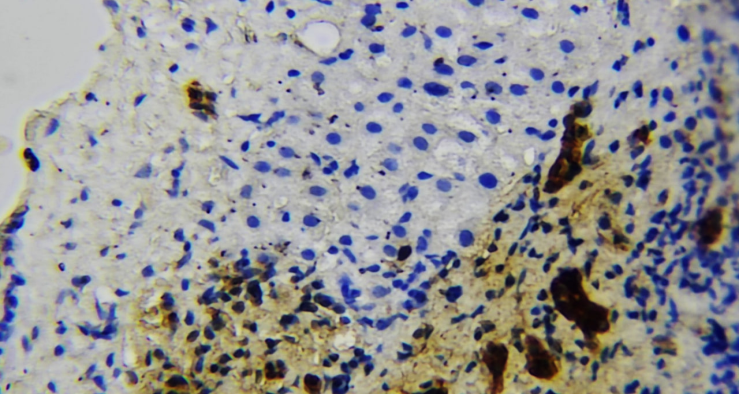 | Figure 1. Correlations between the composition of groundwater and changes in hepatocytes and vascular structures of the liver |
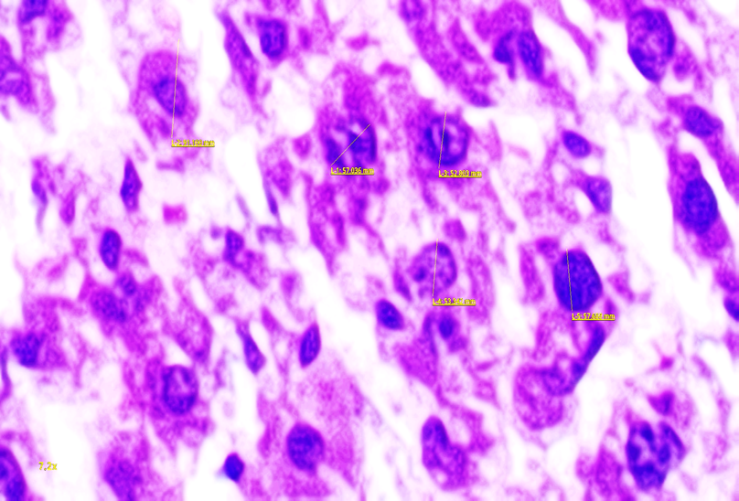
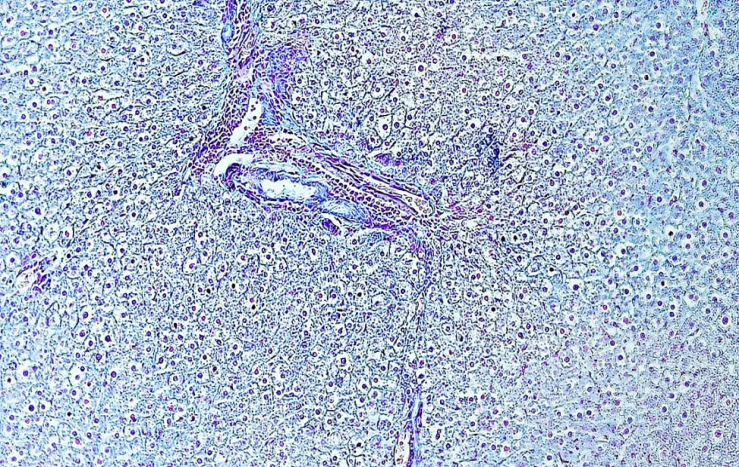 | Figure 3. Liver tissue in 3-month-old rats of the first group, dystrophic changes are visible when using hard water, signs of vacuolar dystrophy of hepatocytes. Stained with alcian blue. Ob: 10x20 |
4. Conclusions
- Thus, an in-depth study of morphological changes in the liver when consuming hard water made it possible to identify potential risks and propose preventive measures for the population living in regions with increased water hardness. Immunohistochemical analysis of the liver revealed changes in the expression of apoptosis and inflammation markers, indicating possible adaptive-compensatory mechanisms.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML