-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(2): 419-421
doi:10.5923/j.ajmms.20251502.31
Received: Jan. 22, 2025; Accepted: Feb. 17, 2025; Published: Feb. 28, 2025

Surgical Treatment of Spastic Syndrome of the Lower Limbs in Children with Cerebral Palsy
Zekriyaev Normurod1, Yugay Igor1, Shoyunusov Sarvar2
1Republican Specialized Scientific Practical Medical Center of Neurosurgery, Uzbekistan
2Tashkent Pediatric Medical Institute, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Childhood Cerebral Palsy (CP) occurs due to brain damage during the perinatal period. CP is characterized by impaired motor functions, increased muscle tone, and other symptoms. Selective dorsal rhizotomy is the most effective treatment method for spastic forms of CP. In this article, we present our experience with rhizotomy. In this article, we present our experience performing selective dorsal rhizotomy (SDR) in 18 patients. The effectiveness of our surgical technique is demonstrated by the postoperative regression of the spastic syndrome.
Keywords: Childhood Cerebral Palsy, Selective dorsal rhizotomy, Spasticity, Diplegia, Tetraparesis
Cite this paper: Zekriyaev Normurod, Yugay Igor, Shoyunusov Sarvar, Surgical Treatment of Spastic Syndrome of the Lower Limbs in Children with Cerebral Palsy, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 419-421. doi: 10.5923/j.ajmms.20251502.31.
1. Introduction
- Cerebral palsy is a serious neurological disorder in children, occurring in 2 per 1,000 newborns and leading to lifelong disability in affected individuals [1]. Among the various factors contributing to disability in patients with cerebral palsy, spasticity in the limbs plays a significant role [1,2]. This condition affects nearly 75% of patients with cerebral palsy [3,4], hindering motor tasks in daily life and leading to muscle contractures and orthopedic deformities in growing children. Selective dorsal rhizotomy (SDR) has been shown to be more effective than other interventions in reducing spasticity in patients with cerebral palsy, thereby improving motor activity and alleviating orthopedic deformities [5,6]. Since 2016, the Republican Scientific and Practical Medical Center of Neurosurgery has been performing surgical interventions on peripheral nerves to relieve limb spasticity. Since 2020, surgeries on the intradural roots of the spinal cord conus, namely SDR, have been introduced. Currently, several surgical techniques for SDR are in use [6].
2. Materials and Methods
- Since 2023, we have performed selective dorsal rhizotomy (SDR) on 18 patients with cerebral palsy. Among them, 12 patients were diagnosed with spastic diplegia, while 6 had spastic tetraparesis. The group included 10 boys and 8 girls, aged from 3 to 18 years. Surgical interventions were carried out using a Carl Zeiss (Germany) operating microscope with a “face-to-face” assistant module and a 32-channel intraoperative neuromonitoring system (Inomed, Germany) with free-run EMG and bipolar stimulation-based root identification.A crucial factor for performing SDR was the meticulous selection of patients. The primary candidates for SDR were children with spastic diplegia who had either mild spasticity in the upper limbs or none at all. During the patient evaluation, we ensured that motor impairments had originated in infancy and had shown steady improvement in early childhood rather than progressive deterioration. Preterm birth was considered a significant factor in selecting SDR candidates. Neurological examination findings determined whether spasticity was the primary and sole cause of muscle hypertonia and whether it significantly impeded motor functions such as sitting, crawling, standing, and walking. Additionally, we conducted a thorough assessment of the severity of orthopedic deformities and their impact on the patient’s motor activity.All patients underwent MRI of the brain and the conus region of the spinal cord. Spinal cord MRI was essential for determining the surgical intervention level and ruling out other pathologies. We considered SDR appropriate for children over the age of 3, as cerebral palsy type and spasticity patterns cannot be reliably diagnosed at a younger age. Dystonia and hyperkinesias accompanying spasticity were regarded as unfavorable factors, as their manifestations tend to worsen postoperatively due to reduced spasticity. Dystonia typically becomes clinically apparent by the age of 5, and in some cases by 10, with a progressive course.Exclusion criteria included patients with severe basal ganglia damage detected on MRI, which was considered a contraindication due to associated rigidity; patients with a history of orthopedic surgery in the past six months; those with severe fixed joint deformities; and severe scoliosis, which was considered a relative contraindication. Additionally, children with increased muscle tone due to severe hydrocephalus, intrauterine or neonatal infections, or head trauma were excluded from the study.
3. Results and Discussion
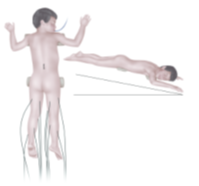
- Anesthesia Considerations. Since the surgical procedure is performed under continuous intraoperative neuromonitoring, the use of muscle relaxants and inhalation anesthetics is excluded during the main stage of the operation. These agents suppress electrical and motor muscle responses during nerve root stimulation. At this stage of the procedure, anesthesia was maintained using propofol, thiopental, and fentanyl.Surgical Technique Features. The patient was positioned prone on the operating table with the head end slightly lower than the lumbar region. This positioning allowed cerebrospinal fluid to accumulate proximally, minimizing its loss from the surgical site in the lumbar region. Needle electrodes were placed bilaterally by neurophysiologists in the adductor muscles, anterior and posterior thigh muscle groups, tibialis anterior muscle, medial head of the gastrocnemius muscle, and plantar foot muscles in preparation for intraoperative EMG studies (Figure 1).1. Prone positioning of the patient.2. Laminectomy at the level of the lower border of the conus.3. Placement of sensory nerve roots on a silicone cushion.4. Direct bipolar stimulation using a probe.5. Partial transection of the sensory root after determining the severity according to the Grade scale.
 | Figure 1. Plantar foot muscles in preparation for intraoperative EMG studies |
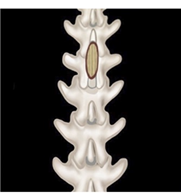 | Figure 2. Ultrasound verification of the conus position after laminectomy |
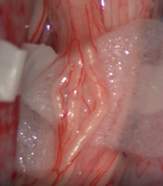 | Figure 3. The suspected sensory root bundles were suspended on a rubber cushion on each side |
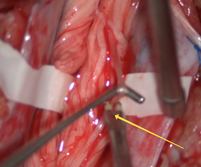 | Figure 4. A probe stimulator delivered single continuous rectangular pulses of 0.1 ms duration at 2 Hz with a current intensity of 0.1 mA (up to 0.4 mA) to individual roots |
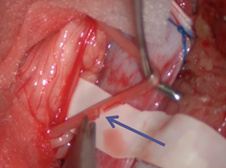 | Figure 5. Grade 1 indicated that root transection was unnecessary |
4. Conclusions
- 1. In spastic diplegia, observed in 18 of our patients, the surgical outcome was complete elimination of lower limb spasticity without a decline in muscle strength. In contrast, partial recurrence of spasticity (up to 2 points on the Ashworth scale) was noted in six patients with spastic quadriplegia after six months.2. The combination of selective dorsal rhizotomy (SDR) and appropriate rehabilitation significantly improves motor skills within 3–6 months after surgery.3. The proposed surgical technique does not require extensive laminectomy and does not lead to spinal instability in the long-term postoperative period.4. Potential complications, such as transient dysesthesia in certain lower limb dermatomes, were temporary and did not affect the timing of motor recovery.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML