-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(2): 362-366
doi:10.5923/j.ajmms.20251502.20
Received: Dec. 12, 2024; Accepted: Jan. 8, 2025; Published: Feb. 14, 2025

Significance of VWF Rs1063857 Gene Polymorphism in the Development of Renal Dysfunction in Patients with Chronic Heart Failure
Zakirova Gulnoza1, Masharipova Dilyafruz1, Boboyev Qodirjon2
1SI "Republican Specialized Scientific and Practical Medical Center of Therapy and Medical Rehabilitation", Tashkent, Uzbekistan
2Republican Specialized Scientific and Practical Medical Center of Hematology, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chronic heart failure (CHF) in most cases leads to multiple organ complications, among which one of the most important is renal dysfunction. The von Willebrand factor (VWF) gene, known for its role in blood coagulation and endothelial function, is involved in various cardiovascular diseases. The rs1063857 polymorphism in the VWF gene may affect renal failure and lead to the development of renal dysfunction in patients with CHF. Von Willebrand factor (VWF) plays a central role in hemostasis and maintenance of endothelial function. The rs1063857 polymorphism of the VWF gene, which alters protein activity, has been associated with various cardiovascular diseases. However, its role in the development of renal dysfunction in patients with CHF remains poorly understood. In-depth study of this relationship may open up new opportunities for early diagnosis and targeted treatment. The aim of this study was to determine the association of the rs1063857 polymorphism in the VWF gene with renal dysfunction in patients with CHF. Blood samples were collected for genotyping of the rs1063857 polymorphism in the VWF gene for polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis in patients with CHF. Renal function was assessed based on the estimated glomerular filtration rate (eGFR) and creatinine levels. To identify potential associations with renal dysfunction, genotype frequencies (C/C, C/T, T/T) of this locus were analyzed, and logistic regression was used to calculate renal failure rates for different genotypes, adjusted for factors such as age, gender, and CHF severity.
Keywords: Chronic heart failure (CHF), Kidney dysfunction (KD), Von Willebrand factor (VWF) gene rs1063857 polymorphism, Genetic association, Renal failure
Cite this paper: Zakirova Gulnoza, Masharipova Dilyafruz, Boboyev Qodirjon, Significance of VWF Rs1063857 Gene Polymorphism in the Development of Renal Dysfunction in Patients with Chronic Heart Failure, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 362-366. doi: 10.5923/j.ajmms.20251502.20.
Article Outline
1. Introduction
- Chronic heart failure (CHF) is high illness and death indicators with separate standing main global health storage is one of the problems in the system. CHF CJD often leads to a cascade of multi-organ complications, with renal dysfunction being one of the most severe and widespread complications.The interaction between heart failure and renal failure, known as cardiorenal syndrome, complicates patient management and worsens outcomes, highlighting the need to better understand the factors that lead to decline in renal function in patients with CHF.The interaction of heart and kidney failure, known as cardiorenal syndrome, complicates patient management and worsens outcomes, highlighting the need for a better understanding of the factors that lead to decline in renal function in patients with CKD. Although clinical factors such as blood pressure, diabetes, and serum creatinine levels play an important role, recent studies have highlighted the importance of genetic predisposition in influencing renal function in these patients [1,2,3,4].While clinical factors such as blood pressure, diabetes and CHF levels play an important role, recent studies have highlighted the importance of genetic predisposition in influencing kidney function in these patients. [1,3,6,7].The VWF gene has attracted attention for research because of its central role in blood coagulation and endothelial function in cardiovascular diseases. As a key player in the vascular system, VWF helps maintain hemostasis, and its expression or function is associated with various cardiovascular and renal pathologies. In particular, the VWF gene rs1063857 polymorphism is associated with altered VWF activity, which may affect vascular and renal complications in patients with CHF. The rs1063857 polymorphism, a single nucleotide change, strongly affects the stability and function of VWF, which in turn may affect endothelial status and renal microcirculation, which are major factors in the development of renal dysfunction in CHF [9].To date, several studies have investigated the relationship between the VWF gene rs1063857 polymorphism and renal dysfunction in patients with CHF. Understanding this association may shed light on the underlying genetic factors of cardiorenal syndrome, allowing for more precise risk profiling and early detection. Furthermore, identifying a genetic marker associated with renal failure in CHF may open up treatment options aimed at preserving renal function and improving overall patient outcomes [5].Many risk factors for the development of CHF, including advanced age, obesity, coronary heart disease (CHD), type 2 diabetes mellitus (T2DM), and renal failure, contribute to the development of a systemic inflammatory state [2,8]. Diastolic dysfunction, CHF, and left ventricular (LV) fibrosis result from this pre-inflammatory state [11].Recent studies have shown that serum biomarkers are important for the development of OS, which is preserved in the development of CHF. As a result, serum biomarkers have been used as a tool for disease prediction and diagnosis. These serum biomarkers include: matrix metalloproteinases (MMP-1, -2, -8, -9), tissue inhibitor of MMP-1 and MMP-2, procollagen type I/II propeptide, procollagen and N-terminal intact procollagen I, terminal telopeptide of collagen type I, tumorigenic factor 2, galectin-3 (Gal-3), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Elevated levels of IL-6, TNF-α, and CRP, along with MMP-2, MMP-9, and Gal-3, were associated severity with the patients with CHF with preserved EF in our previous study. These biomarkers may be useful for the early diagnosis of patients with CHF with preserved EF [4,11].The correlation between the VWF gene rs216311 polymorphism and chronic heart failure (CHF) and venous thrombosis is of increasing interest, given the role of vWF in thrombogenesis. Studies suggest that variations in VWF gene polymorphisms such as rs216311 may influence the risk of thrombosis by altering VWF activity, which may affect thrombus formation and vascular repair, which are important in both heart failure and thrombotic events [10]. This study aimed to characterize the VWF gene rs1063857 polymorphism and assess its association with renal dysfunction in patients with CHF. By studying genotype frequencies and their association with renal function markers, we aim to determine whether this polymorphism serves as a genetic risk factor for renal failure in this population. This knowledge may ultimately help to develop medical approaches to manage renal health in patients with CHF.The purpose of the studyThe main goal of the study is to determine the possibility of the VWF gene rs1063857 polymorphism affecting the formation of pathology in patients with impaired renal function. This, in turn, will help to understand the role of the von Willebrand factor gene in kidney diseases and its significance in the clinical course of the disease.
2. Material and Methods
- 200 patients with chronic heart failure were included in the study, 110 of whom had a CFR>60 ml/min/1.73 m2 and 90 patients with a CFR<60 ml/min/1.73 m2. The control group consisted of 120 conditionally healthy unrelated donors (of Uzbek nationality), who were matched by sex and age to the studied group of patients (p>0.05) and did not have a history of CHF. Detection of the rs1063857 polymorphism in the von Willebrand factor (VWF) gene was performed using test systems from NPF Litekh LLC (Russia) according to the manufacturer's described protocol.For the isolation of DNA from peripheral blood, the AmpliPrime RIBO-prep reagent kit (AmpliSens, Russia) was used. For standard PCR, the polymorphism detection kit from LLC NPF Litekh (Moscow) was used. PCR analysis was performed using RotorGeneQ (QUAGEN, Germany) thermal cyclers.To assess the prevalence of genotype deviation of the studied DNA polymorphisms, the available genetic data for the canonical Hardy-Weinberg model were analyzed using the computer program “GenePop” (“Genetics of Population”) available on the Internet (http://wbiomed.curtin.edu.au/genepop).The software package “OpenEpi 2009, Version 9.3” was used to calculate the obtained data.
3. Research Results and Discussion
- In the studied samples of patients and controls, the true prevalence of genotypes of a given locus in both groups corresponds to that theoretically expected in Hardy-Weinberg equilibrium. Analysis of the frequency of the VWF gene rs1063857 polymorphism in the main group shows that subgroups with different CFT and the control sample are prone to differences in the frequency of alleles and genotypic variants.In the main and control samples of patients, the frequency of the C and T alleles was 73.8% and 26.3%, and 80.0% and 20.0%, respectively (χ2=3.2 and р=0.1) (diagram 1).
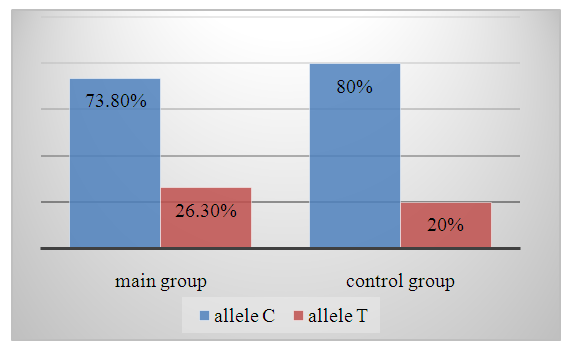 | Diagram 1. Frequency of VWF gene alleles in the main and control groups |
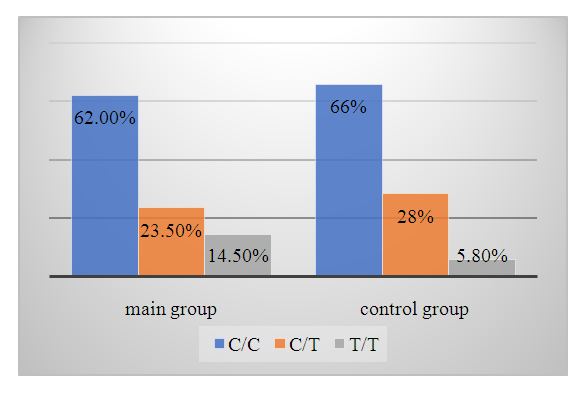 | Diagram 2. Frequency of VWF gene genotypes in the main and control groups |
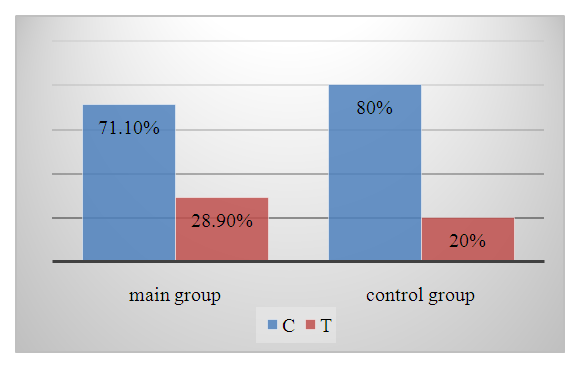 | Diagram 3. Frequency of VWF gene еGFT<60 ml/min/1.73 m2 alleles in the main and control groups |
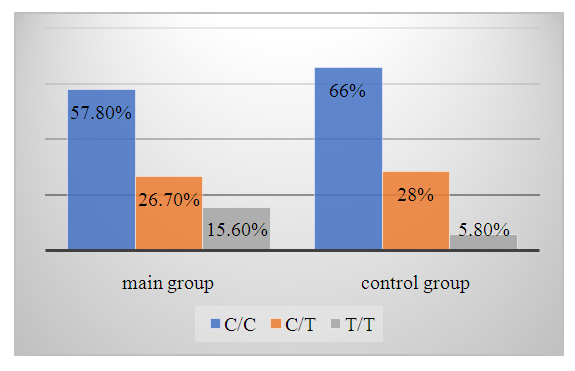 | Diagram 4. The frequency of VWF gene еGFT<60 ml/min/1.73 m2 genotypes in the main and control groups |
4. Discussion
- In recent years, genetic research has made significant progress in understanding the mechanisms of cardiorenal syndrome. The rs1063857 polymorphism of the VWF gene has been recognized as an important factor affecting endothelial and hemostatic functions. This polymorphism affects the stability and activity of the von Willebrand factor, which can provoke microvascular disorders, especially in the kidneys. This is of particular importance for patients with chronic heart failure (CHF), in whom renal microcirculation disorders exacerbate systemic inflammation and contribute to the progression of renal dysfunction.This study investigated the association between the VWF gene rs1063857 polymorphism and renal dysfunction in patients with chronic heart failure (CHF). Our results suggest that certain genotypes of the VWF gene rs1063857 polymorphism (particularly T/T) may play a role in increasing the risk of renal dysfunction. In particular, the association of this genotype with low glomerular filtration rate (GFR) as shown in the study further increases the genetic significance of this polymorphism in renal disease.The National Kidney Foundation (2021) study on cardiorenal syndrome identified the impact of various systemic factors on renal dysfunction in patients with CKD. Genetic predisposition and the above polymorphisms play an important role [4].In addition, factors such as high blood pressure and diabetes mellitus may also contribute to the development of renal dysfunction in patients with CHF. The work of De Caterina and Libby (2019) highlighted the impact of endothelial dysfunction on the development of cardiac and renal pathologies [3].Endothelial dysfunction associated with changes in VWF activity is a key mechanism of renal injury. The rs1063857 polymorphism, especially the T/T genotype, is associated with increased levels of proinflammatory cytokines such as IL-6 and TNF-α, which enhances pathological processes in the kidneys. This is supported by research data on the role of the endothelium in cardiorenal syndrome [4].The work of Wang et al. (2020) highlighted the relationship between the VWF gene and inflammatory mechanisms in the cardiovascular system. This study, together with the current study, will help to better understand the impact of genetic factors on renal microcirculation [11].A study by Song et al. (2021) showed that the VWF gene rs216311 polymorphism is associated with venous thrombosis, indicating that these genetic changes are important not only for cardiorenal syndrome but also for thrombogenesis in heart failure. This study also paid special attention to the effect of the polymorphism on endothelial status [10].According to the American Heart Association, the pathophysiology of cardiorenal syndrome is multifaceted, and genetic factors play an important role in it. The effect of the VWF gene rs1063857 polymorphism on renal function shown in this study is consistent with the studies of Lü et al. (2022) on the relationship between von Willebrand factor and vascular diseases. They found that many polymorphisms of this gene affect blood coagulation and endothelial function in the body [3].In support of the findings of the present study, the work of Wang et al. (2020) highlighted the contribution of inflammatory mechanisms, in particular the influence of VWF, to the pathophysiology of heart and kidney failure [11]. In addition, recent studies by Kim et al. (2023) demonstrated that genetic predisposition, including VWF gene polymorphisms, can serve as a basis for risk prediction and individualization of treatment approaches in patients with CHF [5].Therefore, the association between the rs1063857 polymorphism and renal dysfunction in patients with SJS suggests that genetic alterations in VWF may play a role in the pathophysiology of cardiorenal syndrome in patients with CHF. The VWF gene encodes von Willebrand factor, an important component of endothelial function and hemostasis.The association between the T/T genotype of the rs1063857 polymorphism and renal dysfunction may also be related to alterations in VWF activity. Alterations in VWF function may increase susceptibility to microvascular complications, including renal microcirculation. This is consistent with previous studies linking VWF activity to endothelial injury and inflammation, both of which are important in the development of renal failure in patients with CHF.The identification of the VWF gene rs1063857 polymorphism as a risk factor for renal dysfunction in patients with CHF has important clinical implications. Genotyping for this polymorphism may help identify patients with CHF who are at high risk of renal failure, which may lead to early diagnosis and appropriate management. Preventive measures such as monitoring renal function, use of renal protective drugs, and lifestyle changes may be particularly beneficial for carriers of the T/T genotype.In conclusion, this study demonstrates a significant association between the VWF gene rs1063857 polymorphism and renal dysfunction in patients with CHF, suggesting that this genetic variation may serve as a potential risk marker for renal failure in this population. These results highlight the importance of genetic screening in the management of CHF, as identifying patients with a genetic predisposition to renal dysfunction may allow for more targeted and effective interventions.
5. Summary
- This study suggests that a high frequency of the T allele of the VWF gene rs1063857 polymorphism may be a risk marker for patients with chronic kidney disease. In patients with certain genotypes, especially T/T carriers, renal dysfunction may be associated with an increased risk in certain situations, including those with low CHF. Identification of the rs1063857 polymorphism as a potential genetic risk factor for renal dysfunction in patients with CHF has important prognostic implications for clinical practice. Genotyping for this polymorphism may help in the early identification of CHF patients at high risk of renal complications, and provide an individualized approach to treatment strategy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML