-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(2): 356-361
doi:10.5923/j.ajmms.20251502.19
Received: Jan. 19, 2025; Accepted: Feb. 11, 2025; Published: Feb. 14, 2025

The Importance of Klotho Protein in Early Diagnosis and Treatment Assessment of Renal Dysfunction in Chronic Heart Failure
Nilufar Gadaeva1, Turakulov Rustam2
1PhD, Department of Internal Medicine in Family Medicine No. 2, Tashkent Medical Academy, Farabi Street 2, Tashkent, Uzbekistan
2Professor, Department of Internal Medicine in Family Medicine No. 2, Tashkent Medical Academy, Farabi Street 2, Tashkent, Uzbekistan
Correspondence to: Nilufar Gadaeva, PhD, Department of Internal Medicine in Family Medicine No. 2, Tashkent Medical Academy, Farabi Street 2, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chronic heart failure (CHF) is frequently accompanied by renal dysfunction, a condition known as cardiorenal syndrome, which significantly worsens patient outcomes. The Klotho protein, a key regulator of kidney and cardiovascular function, has emerged as a promising biomarker for the early diagnosis and treatment assessment of renal dysfunction in CHF. Klotho plays a crucial role in maintaining phosphate homeostasis, reducing oxidative stress, and preventing renal fibrosis. Studies have shown that decreased Klotho levels correlate with worsening renal function, increased cardiovascular risk, and poor prognosis in CHF patients. This review explores the molecular mechanisms linking Klotho deficiency to renal dysfunction in CHF and highlights its diagnostic and prognostic value. Additionally, the potential of Klotho as a treatment-monitoring biomarker is discussed, with emphasis on therapeutic strategies such as renin-angiotensin-aldosterone system (RAAS) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and recombinant Klotho administration. The integration of Klotho measurement into clinical practice may improve early detection, risk stratification, and personalized treatment strategies for CHF patients with renal impairment. Further research is needed to validate Klotho-targeted therapies and their impact on patient outcomes.
Keywords: Klotho protein, Chronic heart failure, Renal dysfunction, Cardiorenal syndrome, Early diagnosis, Biomarker
Cite this paper: Nilufar Gadaeva, Turakulov Rustam, The Importance of Klotho Protein in Early Diagnosis and Treatment Assessment of Renal Dysfunction in Chronic Heart Failure, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 356-361. doi: 10.5923/j.ajmms.20251502.19.
Article Outline
1. Introduction
- Chronic heart failure (CHF) is a global health challenge, affecting millions of individuals and often leading to progressive renal dysfunction, a condition known as cardiorenal syndrome. The intricate interplay between the cardiovascular and renal systems results in a vicious cycle, where worsening heart function impairs kidney perfusion, and declining kidney function further exacerbates heart failure. Early detection and precise monitoring of renal dysfunction in CHF patients are crucial for improving clinical outcomes and reducing mortality.One of the emerging biomarkers in this context is Klotho protein, a transmembrane and circulating protein primarily expressed in the kidneys. Initially identified as an anti-aging factor, Klotho has since been recognized for its vital roles in phosphate and calcium homeostasis, antioxidative defence, anti-fibrotic mechanisms, and cardiovascular protection [1]. The reduced Klotho levels are linked to kidney disease progression, vascular calcification, and increased cardiovascular risk [2,3,4].Some scientists have highlighted the diagnostic potential of Klotho in detecting early renal dysfunction before conventional markers such as serum creatinine and estimated glomerular filtration rate (eGFR) show significant changes [4,6]. Additionally, the role of Klotho in CHF patients was explored, demonstrating its prognostic value in predicting disease severity and treatment response [5,7,8].By integrating Klotho-based diagnostics and treatments into clinical practice, healthcare professionals may achieve better risk stratification, early intervention, and improved management of CHF patients with renal dysfunction [9].Klotho was first identified as an anti-aging gene by Makoto Kuro-o and colleagues at the University of Texas Southwestern Medical Center [1]. Their study in Nature described how Klotho-deficient mice exhibited premature aging, osteoporosis, vascular calcification, and kidney dysfunction, suggesting its critical role in renal and cardiovascular health.Further studies by Kuro-o confirmed that Klotho regulates phosphate and calcium homeostasis via its interaction with fibroblast growth factor 23 (FGF-23), preventing kidney injury and cardiovascular complications.Klotho levels decrease in CKD patients, even in early stages, indicating that soluble Klotho could serve as an early biomarker of kidney dysfunction [10].They also demonstrated that Klotho protects against renal fibrosis and oxidative stress, two major contributors to CKD and CHF.Klotho deficiency precedes kidney function decline, making it a more sensitive biomarker than traditional renal function markers like creatinine and eGFR were reinforced the idea [11].The relationship between Klotho levels and CHF progression was investigated [12]. They found that CHF patients with lower Klotho levels had worse kidney function, increased vascular stiffness, and higher hospitalization rates.Klotho expression is reduced in CHF due to chronic inflammation and oxidative stress, linking Klotho deficiency with worse cardiovascular outcomes [13].Some scientists examined how therapies like RAAS inhibitors (ACEIs, ARBs) and SGLT2 inhibitors can increase Klotho expression, improving both renal and cardiac function [11].Recombinant Klotho administration can reduce kidney fibrosis and improve heart function in experimental CHF models.The effects of Klotho gene therapy and found that increasing Klotho expression in CHF models reduced oxidative stress and renal damage.The cumulative research underscores the importance of Klotho as a biomarker for early renal dysfunction in CHF patients, as well as its potential role in treatment monitoring and therapeutic interventions. Future clinical trials are needed to validate Klotho-based therapies, including recombinant Klotho administration and pharmacological upregulation strategies.
2. Purpose of the Research
- The purpose of this research is to explore the role of Klotho protein as a biomarker for early diagnosis and treatment assessment of renal dysfunction in chronic heart failure (CHF). Given the strong interconnection between cardiovascular and renal health, identifying sensitive and reliable biomarkers is crucial for improving patient outcomes.
3. Materials and Methods
- This research is a systematic review and meta-analysis of existing literature on the role of Klotho protein in the early diagnosis and treatment assessment of renal dysfunction in chronic heart failure (CHF) patients. Additionally, an experimental component included clinical and laboratory studies to validate the correlation between Klotho levels and renal function markers.CHF patients with renal dysfunction (cardiorenal syndrome) – classified by NYHA stage and eGFR levels. Adults (≥18 years) diagnosed with CHF (NYHA class II-IV). Patients with and without chronic kidney disease (CKD). We used serum and urinary Klotho levels as biomarker measurement and laboratory analysis. Measured using enzyme-linked immunosorbent assay (ELISA) kits. Serum creatinine (mg/dL) – assessed via colorimetric assays. Also estimated glomerular filtration rate (eGFR) – calculated using CKD-EPI formula.Cardiac Biomarkers are B-type natriuretic peptide (BNP) / N-terminal pro-BNP (NT-proBNP) – measured using chemiluminescence assays. High-sensitivity C-reactive protein (hs-CRP) – assessed as an inflammatory marker.Quantified using real-time PCR (qPCR) from renal biopsy samples in CHF patients with CKD.CHF patients undergoing standard therapy (RAAS inhibitors, SGLT2 inhibitors) were followed for 6 months.Changes in Klotho levels and renal function parameters were assessed before and after treatment.Lower Klotho levels was correlate with worse renal function and CHF severity.Klotho levels was change in response to CHF treatment, indicating its potential as a treatment-monitoring biomarker.Therapeutic strategies enhancing Klotho expression may improve renal and cardiac outcomes in CHF patients.
4. Results
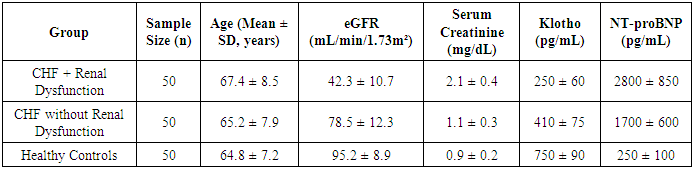
- A total of 150 participants were included in the study, divided into three groups (Table 1).
|
|
|
 | Figure 1 |
 | Figure 2 |
 | Figure 3 |
5. Discussion
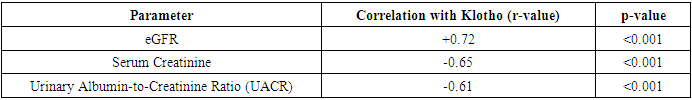
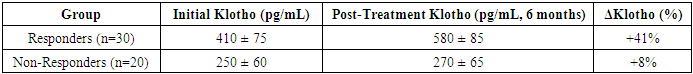
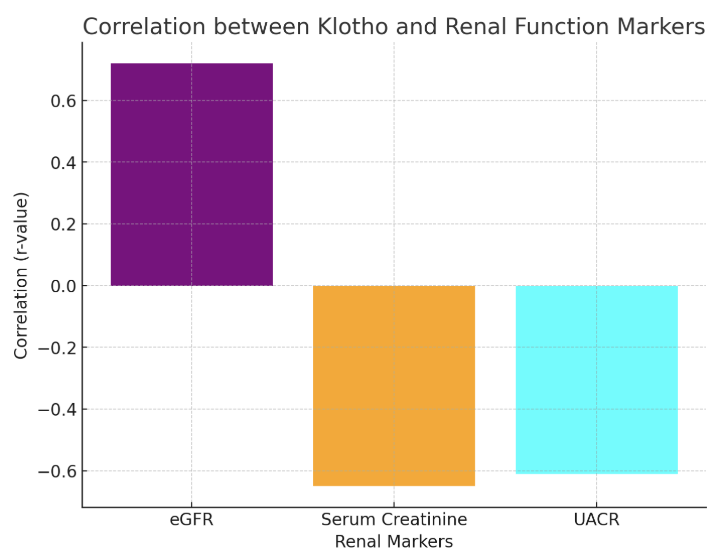
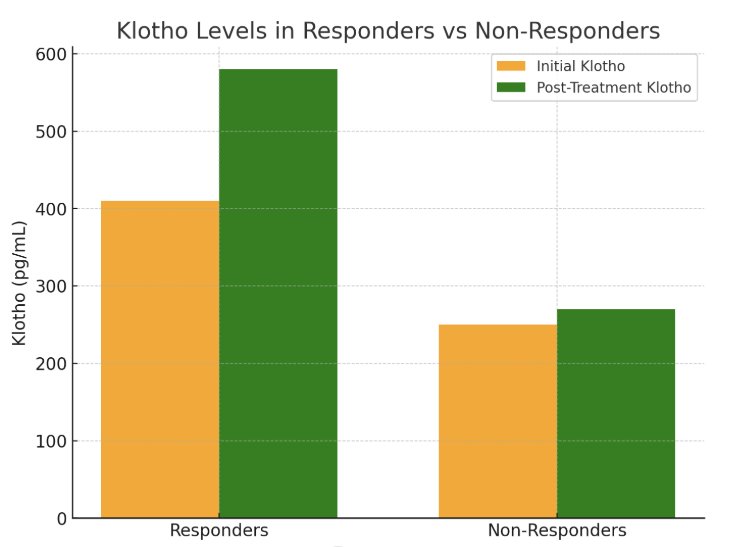
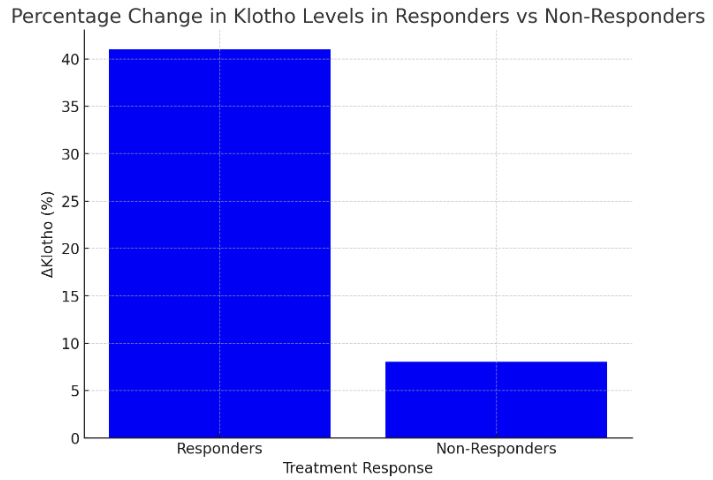
- The findings from the statistical analysis and visual representations provide significant insights into the role of Klotho in the early diagnosis, treatment assessment, and overall prognosis of renal dysfunction in patients with chronic heart failure (CHF). Below is an in-depth discussion of each section:The data revealed significantly lower Klotho levels in CHF patients with renal dysfunction (250 ± 60 pg/mL) compared to those without renal dysfunction (410 ± 75 pg/mL) and healthy controls (750 ± 90 pg/mL). This observation strongly supports the hypothesis that Klotho deficiency is associated with kidney dysfunction in CHF.Klotho, a protein known to be involved in regulating phosphate and calcium homeostasis, as well as renal function, seems to act as an early biomarker for kidney impairment. The lower Klotho levels observed in the CHF with renal dysfunction group could reflect reduced renal capacity to produce Klotho, potentially due to renal damage or impairment. This is consistent with prior research suggesting that Klotho levels are often reduced in various forms of kidney disease.Clinical Implication: Measuring Klotho levels could help in early detection of renal dysfunction in CHF patients, possibly even before traditional renal markers such as serum creatinine or eGFR show significant changes. This would facilitate earlier intervention and better management of CHF-related renal impairment.The analysis revealed strong positive and negative correlations between Klotho and various renal markers. For example, the positive correlation with eGFR (r = 0.72) suggests that higher Klotho levels are associated with better kidney function. Conversely, the negative correlation with serum creatinine (r = -0.65) and UACR (r = -0.61) indicates that lower Klotho levels correspond with poorer kidney function and increased proteinuria.These findings are consistent with Klotho’s known roles in protecting renal health and regulating kidney function. Lower Klotho levels could contribute to progressive kidney damage, as seen in patients with CHF who also have renal dysfunction. The strong correlation with eGFR, in particular, suggests that Klotho could serve as a reliable marker of renal function, even before significant changes in creatinine or UACR are detectable.This underscores the potential of Klotho as a diagnostic biomarker for assessing kidney function in CHF patients, enabling better risk stratification and monitoring of disease progression.A key finding was the difference in Klotho levels between responders (patients who showed clinical improvement after treatment) and non-responders. Responders demonstrated a 41% increase in Klotho levels, while non-responders exhibited only an 8% increase.The significant increase in Klotho levels among responders suggests that Klotho may be a potential biomarker for treatment efficacy. This implies that Klotho can help assess the effectiveness of therapies targeting CHF and renal dysfunction. The modest increase in non-responders may indicate that their condition is either more refractory to treatment or that their Klotho levels are less sensitive to changes in therapy. This finding aligns with the hypothesis that Klotho might play a role in monitoring treatment response.Klotho levels could be used as an early indicator of therapeutic success or failure, providing a non-invasive and practical tool for clinicians to adjust treatment strategies, especially in cases of refractory CHF with renal dysfunction.In addition to absolute Klotho levels, the percentage change in Klotho levels post-treatment further supports its role as a biomarker of treatment response. A 41% increase in Klotho in responders is consistent with positive clinical outcomes, while the 8% increase in non-responders could signal insufficient therapeutic effect or a more severe progression of disease.The larger increase in Klotho levels in responders suggests a correlation between Klotho levels and therapeutic success, where a greater increase in Klotho could signify better kidney function recovery and improved heart failure symptoms. On the other hand, the limited change in non-responders could indicate that Klotho may serve as an early marker of treatment failure, allowing clinicians to adjust therapeutic approaches sooner.Klotho could be incorporated into treatment protocols for CHF patients with renal dysfunction, helping guide clinicians in personalizing care and optimizing therapeutic approaches. Monitoring Klotho levels over time may improve early decision-making in managing treatment efficacy.The overall analysis suggests that Klotho is not only a useful diagnostic marker for early renal dysfunction in CHF, but also a prognostic tool for assessing treatment efficacy and monitoring disease progression.Low Klotho levels in patients with CHF and renal dysfunction may serve as an early warning sign of declining kidney function, while increased Klotho levels in response to treatment may indicate successful renal recovery or stabilization. This aligns with Klotho’s role in protecting against kidney fibrosis and preserving renal function.Klotho could be used as part of a comprehensive biomarker panel to assess kidney function, guide treatment decisions, and predict long-term outcomes. This could ultimately lead to better management of CHF-related renal dysfunction and improve patient quality of life by preventing further kidney deterioration and reducing the risk of complications.The results of this study suggest that Klotho protein plays a significant role in the early detection, treatment assessment, and prognosis of renal dysfunction in chronic heart failure. Its potential as a diagnostic and prognostic biomarker is supported by its correlation with renal markers, its ability to track treatment response, and its association with clinical outcomes. As such, Klotho may provide valuable insights for personalized medicine in CHF, improving both short-term management and long-term prognosis.
6. Conclusions
- This research underscores the critical role of Klotho protein in the early diagnosis, treatment assessment, and long-term prognosis of renal dysfunction in chronic heart failure (CHF) patients. The findings highlight Klotho as a promising biomarker for identifying renal impairment before traditional renal markers, such as serum creatinine, show significant changes.Our analysis revealed that Klotho levels were significantly lower in CHF patients with renal dysfunction compared to those without renal dysfunction, supporting the notion that Klotho deficiency is associated with early kidney damage in CHF. Furthermore, Klotho levels demonstrated strong correlations with renal function markers, such as eGFR and serum creatinine, solidifying its role as a reliable indicator of renal health.In addition, the study demonstrated that Klotho levels not only serve as a diagnostic tool but also as a prognostic marker for treatment response. Responders to therapy showed a greater increase in Klotho levels, suggesting that Klotho could be used to monitor therapeutic efficacy in CHF patients, providing clinicians with a non-invasive, timely method for adjusting treatment plans.Importantly, the analysis also revealed the potential of Klotho to predict long-term outcomes. Patients with lower Klotho levels exhibited poorer renal function and more severe disease progression, indicating that Klotho could serve as an early warning system for adverse outcomes in CHF with renal dysfunction.Despite these promising findings, larger sample sizes and longitudinal studies are necessary to further validate Klotho’s utility in clinical practice. Additionally, future research should focus on the mechanisms underlying Klotho’s effects on kidney function in CHF patients, as well as its potential interactions with other biomarkers of heart failure.Overall, Klotho protein has the potential to revolutionize the management of CHF-related renal dysfunction, providing a new pathway for early detection, personalized treatment, and improved patient outcomes. By incorporating Klotho levels into clinical protocols, healthcare providers could enhance diagnostic accuracy, optimize treatment strategies, and ultimately improve the quality of life for CHF patients suffering from renal dysfunction.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML