-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(2): 346-350
doi:10.5923/j.ajmms.20251502.17
Received: Jan. 19, 2025; Accepted: Feb. 9, 2025; Published: Feb. 14, 2025

Genes of the Integrin Family of Platelet Receptors (ITGА2 (C807T) and ITGβ3 (T1565C)): Features of Distribution and Analysis of the Role in Immune Thrombocytopenia
Dilbar Erkinovna Djuraeva1, Dilfuza Saburovna Matkarimova2, Kodirzhon Tukhtaboevich Boboev3
1Termez Branch of Tashkent Medical Academy MoH RUz, Termez, Uzbekistan
2Department of Hematology, Transfusiology and Laboratory Science, Tashkent Medical Academy MoH RUz, Tashkent, Uzbekistan
3Laboratory of Medical Genetics, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
Correspondence to: Dilbar Erkinovna Djuraeva, Termez Branch of Tashkent Medical Academy MoH RUz, Termez, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to analyze the distribution features and the role of platelet receptor integrin genes (ITGA2 (C807T) and ITGβ3 (T1565C)) in immune thrombocytopenia. The study involved 162 adults (median age 53.3±1.4 years), including 82 patients with CLD (1st main group: - 38 with chronic viral hepatitis (CH) and 44 with liver cirrhosis (LC)), who were treated in the therapy department of the Khorezm Regional Multidisciplinary Medical Center (KhRMMC) in the period from 2020 to 2023, and 80 conditionally healthy volunteers (mean age 51.8±1.7 years). SNP genotyping of the TLR4 (-728 GC) and TLR4 (-2272 AG) genes was performed. DNA was extracted from whole blood using the DNA-sorb-B reagent kit (Russia). Genotyping was performed by PCR using a universal reagent kit (Litech, Russia), according to the manufacturer's instructions. For mathematical calculations of the obtained results, the OpenEpi 2009, Version 9.2 statistical software package was used. The distribution of observed and expected genotype frequencies of the studied polymorphic gene was compared in accordance with the Hardy-Weinberg equilibrium (HWE) (P> 0.05), comparative analysis of SNP of the TLR4 (-728 GC) and TLR4 (-2272 AG) genes between the groups of patients and healthy individuals (case-control) was carried out by calculating the χ2 criterion, reliability (P), the odds ratio (OR) and the confidence interval (95% CI). The differences identified were considered reliable at P ≤ 0.05. Taking into account the obtained results, it can be concluded that SNPs of the ITGА2 (C807T) and ITGβ3 (T1565C) genes are not involved in the mechanisms of increasing the risk of immune thrombocytopenia (ITP) development. However, the mutant allele and genotype of the ITGβ3 (T1565C) gene SNP are statistically significantly associated with a severe decrease in the number of platelets in immune thrombocytopenia (ITP).
Keywords: Immune thrombocytopenia, Platelets, Integrins, ITGА2 (C807T), ITGβ3 (T1565C), Risk of development, Severity of thrombocytopenia
Cite this paper: Dilbar Erkinovna Djuraeva, Dilfuza Saburovna Matkarimova, Kodirzhon Tukhtaboevich Boboev, Genes of the Integrin Family of Platelet Receptors (ITGА2 (C807T) and ITGβ3 (T1565C)): Features of Distribution and Analysis of the Role in Immune Thrombocytopenia, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 346-350. doi: 10.5923/j.ajmms.20251502.17.
Article Outline
1. Introduction
- The problem of immune thrombocytopenia (ITP) due to its widespread prevalence and severity of hemorrhagic complications has been of particular interest to scientists for centuries [1]. Studies on the epidemiological characteristics of ITP have shown that the prevalence rates of the disease vary significantly from 4.5 to 20 per 100 thousand people, but the incidence of the disease among the group of hemorrhagic diatheses averages 25%, with half of them having thrombocytopenia accompanied by hemorrhagic manifestations [2,3].An analysis of the periods of study of immune thrombocytopenia shows their importance for the formation of modern views in terminology, understanding the mechanisms of their development, on the basis of which significant progress has been achieved in the diagnosis and treatment of these complex diseases [4,5]. Meanwhile, the question of the causes and conditions that contribute to the development of immune thrombocytopenia remains controversial today.The complexity of the etiological and mechanisms of immune thrombocytopenia allows us to define it as a multifactorial pathology, an important role in the formation of which is given to both exogenous and endogenous development factors, the impact of which leads to the formation of antiplatelet antibodies leading to premature destruction of platelets in immune thrombocytopenia [6,7].As a result of numerous studies, data has been accumulated to date on the high significance of the genetic component in the risk of developing immune thrombocytopenia, which largely determines the severity of its clinical course and the development of formidable complications [8,9].It is known that a large number of genes are involved in platelet differentiation [10,11,12]. Mutations in one of these genes can potentially lead to thrombocytopenia due to decreased formation or shortened lifespan of platelets [13].When analyzing the literature on the involvement of the genetic component in the development and severity of immune thrombocytopenia, numerous studies were found to study the role of SNPs of cytokine genes. However, the results of the analysis of the influence of platelet receptor integrin genes in increasing the risk of immune thrombocytopenia and its severity are few [14,15].The molecular pathogenesis of immune thrombocytopenia has not been fully studied, and no single process can be considered as an unambiguous mechanism for the development of this pathology [16].In turn, conducting molecular genetic analyses of platelet receptor integrin genes (ITGA2 (C807T) and ITGβ3 (T1565C)) in immune thrombocytopenia will improve the understanding of the pathogenetic mechanisms of the disease, as well as develop criteria for predicting the formation, development, clinical course and outcomes of the disease, which will ultimately lead to broad opportunities for improving early diagnosis, the effectiveness of treatment results and the quality of life of patients with immune thrombocytopenia.
2. Main Body
2.1. The Purpose of Our Research
- To analyze the distribution features and role of platelet receptor integrin genes (ITGА2 (C807T) and ITGβ3 (T1565C)) in immune thrombocytopenia.
2.2. Material and Methods of Study
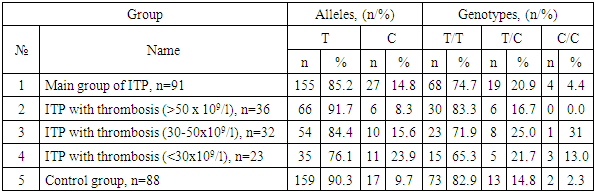
- The study involved 179 adults (median age 38.4±1.8 years), including 91 patients with immune thrombocytopenia (the 1st main group of patients) and 88 healthy volunteers without blood system pathology (the 5th control comparison group). Of the subjects, 48% were female and 52% were male. The 1st main group of patients with immune thrombocytopenia (n=91) was divided into three groups depending on the platelet count: the 2nd group of immune thrombocytopenia with a platelet level of > 50 x 109/l (n=36); the 3rd group of immune thrombocytopenia with a platelet level of 30-50 x 109/l (n=32) andthe 4th group of immune thrombocytopenia with a platelet level of <30 x 109/l (n=23).All patients included in the study were selected randomly as they applied to the Termez Regional Multidisciplinary Medical Center (TRMMC, Republic of Uzbekistan, Termez) in the period from 2021 to 2023.In this study, all examined individuals underwent molecular genetic testing to study the SNP characteristics of the ITGА2 (C807T) and ITGβ3 (T1565C) genes. Genomic DNA was isolated from venous blood leukocytes using the Ribo-Preb kit (Russia). Allelic variants of the ITGА2 (C807T) and ITGβ3 (T1565C) genes were determined using restriction analysis of amplification products of genomic regions. DNA fragment amplification was performed using polymerase chain reaction (PCR) with specific oligonucleotide primers, with an annealing temperature of 600C (Rotor Gene Q, (Quagen, Germany), using “Syntol” test systems (Russia). Genotypes were visualized using electrophoretic separation of restriction products.In this study, all examined individuals underwent molecular genetic testing to study the SNP characteristics of the ITGА2 (C807T) and ITGβ3 (T1565C) genes. Genomic DNA was isolated from venous blood leukocytes using the Ribo-Preb kit (Russia). Allelic variants of the ITGА2 (C807T) and ITGβ3 (T1565C) genes were determined using restriction analysis of amplification products of genomic regions. DNA fragment amplification was performed using polymerase chain reaction (PCR) with specific oligonucleotide primers, with an annealing temperature of 600C (Rotor Gene Q, (Quagen, Germany), using Syntol test systems (Russia). Genotypes were visualized using electrophoretic separation of restriction products.Comparative analysis of allele and genotype frequencies of ITGА2 (C807T) and ITGβ3 (T1565C) gene polymorphisms was performed using Pearson's χ2 criterion, odds ratio (OR) and confidence interval (95% CI). The ratio of genotype frequencies of the studied genes was analyzed for compliance with the Hardy-Weinberg equilibrium. Differences were assessed as statistically significant at P ≤0.05. Statistical calculations of the obtained results were performed using the OpenEpi 2009, Version 9.2 statistical software package.
2.3. Results of the Study
- An assessment of the distribution of SNPs of the genetic markers ITGА2 (C807T) and ITGβ3 (T1565C) for compliance with RHT showed the absence of statistically significant differences between the observed (Ho) and expected (He) frequencies of genotypes in the groups of patients with immune thrombocytopenia and controls (RHT, p>0.05).An assessment of the distribution of SNP genetic markers ITGА2 (C807T) and ITGβ3 (T1565C) for compliance with the Hardy-Weinberg equilibrium showed the absence of statistically significant differences between the observed (Ho) and expected (He) genotype frequencies in the groups of patients with immune thrombocytopenia and controls (p>0.05).The polymorphism of the ITGА2 gene (C807T) was distributed according to the principle of dominance of the main allele and genotype, the maximum frequency of which was observed in the group of patients with immune thrombocytopenia with a mild decrease in platelets. The recessive allele C (19.3%) was most often recorded in the group of healthy people, while the frequency of heterozygote C/T (28.1%) was higher among patients with immune thrombocytopenia with moderate thrombocytopenia, and the mutant genotype T/T among patients with immune thrombocytopenia with severe thrombocytopenia (8.7%) (see Table 1).
|
|
3. Conclusions
- Analyzing the results of the study of the nature of the distribution of SNP of the ITGА2 gene (C807T) among patients with immune thrombocytopenia and healthy people, statistically significant differences between allelic and genotypic variants showing their participation in the risk of immune thrombocytopenia were not established.In addition, analyzing the distribution features of the polymorphic loci of the ITGβ3 gene (T1565C) in groups of patients with immune thrombocytopenia and healthy individuals, no statistically significant association with the risk of immune thrombocytopenia and the studied marker was found. However, a reliable correlation was found between the carriage of weakened mutant alleles C and the C/C genotype with an increase in the risk of severe decrease in the number of platelets in immune thrombocytopenia by 2.9 (χ2=6.7; P=0.01) and 6.5 times (χ2=4.9; P=0.05), respectively.In addition, the analysis revealed a statistically significant decrease in the risk of severe thrombocytopenia compared to moderate thrombocytopenia among carriers of protective T loci by 3.5 times (χ2=5.5; P=0.03) with a tendency to decrease this risk among carriers of the main T/T genotype by 2.7 times (χ2=2.5; P=0.2).Therefore, taking into account the obtained results, it can be concluded that SNPs of the ITGА2 (C807T) and ITGβ3 (T1565C) genes are not involved in the mechanisms of increasing the risk of developing immune thrombocytopenia. However, the mutant allele and genotype of the ITGβ3 (T1565C) gene SNP are statistically significantly associated with a severe decrease in the number of platelets in immune thrombocytopenia.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML