-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(2): 289-291
doi:10.5923/j.ajmms.20251502.04
Received: Dec. 30, 2024; Accepted: Jan. 23, 2025; Published: Feb. 7, 2025

Morphological and Immunohistochemical Changes in the Large Intestine in White Rats with Experimental Pneumonic Fibrosis
Togaev Jurabek Fakhriddinovich
Bukhara Medical Institute named after Abu Ali ibn Sinо, Bukhara, Uzbekistan
Correspondence to: Togaev Jurabek Fakhriddinovich, Bukhara Medical Institute named after Abu Ali ibn Sinо, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article reveals morphological changes in the lymphoid structures of the colon wall of purebred rats with experimental pneumosclerosis. When studying the immune status of rats against the background of prolonged hypoxia in experimental pneumosclerosis, significant disturbances were revealed in the form of a sharp decrease in the number of lymphotsitis. This served as the basis for a comparative analysis of various morphological changes in lymphoid tissue and dynamics in rats, and allowed us to determine structural changes in intestinal tissues.
Keywords: Colon, Pneumosclerosis, Experiment, Hypoxia, Morpholog, Immunohistochemistry, Lung
Cite this paper: Togaev Jurabek Fakhriddinovich, Morphological and Immunohistochemical Changes in the Large Intestine in White Rats with Experimental Pneumonic Fibrosis, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 289-291. doi: 10.5923/j.ajmms.20251502.04.
1. Introduction
- According to the World Health Organization, lung diseases are one of the world's greatest challenges, causing 1/6 of global deaths. The term "pneumosclerosis" defines a pathological condition in which the lung parenchyma undergoes an irreversible process of excessive growth, sclerosis and / or scarring, which is associated with excessive deposition of extracellular matrix components, including collagen. Fibrosis of lung tissue is an irreversible process that can only be prevented or stopped in the early stages [1,2].Interstitial lung diseases (ILDs) are a heterogeneous group characterized by a variety of clinical, radiological and pathological patterns that widely affect the lung parenchyma. Some IPFs are characterized by varying degrees of pneumosclerosis, of which idiopathic pneumosclerosis (idiopathic pulmonary fibrosis, IPF) is the most representative. IPF has the worst prognosis, with a median survival of 2–5 years after diagnosis, making it a major medical problem that has not yet been resolved. IPF is more common in older age, in men than in women, and in the absence of any specific provocation. Pneumosclerosis also represents the final stage of IPF [3,4,5]. Fibroblasts play an important role in tissue repair by proliferating and differentiating into myofibroblasts and by modulating the volume of the extracellular matrix [7,9]. Myofibroblasts produce a denser extracellular matrix than fibroblasts, and the presence of smooth muscle actin leads to spatial reorganization of collagen fibrils. Thickening and densification of lung tissue impedes gas exchange and ultimately leads to decreased lung function [8,10,11].The aim of the research. To determine the dynamics of the condition and characteristics of lymphoid tissue in various parts of the colon wall in experimental pneumosclerosis.
2. Materials and Methods
- The experiment involved 120 mature white mongrel rats weighing 220–240 g and aged 5–6 months. All animals were divided into experimental and control groups. Before the experiment, the rats were acclimatized for 7 days in standard conditions corresponding to the sanitary standards of Uzbekistan. The conditions included a temperature of 20–24°C, humidity of 50–70%, and a 12-hour light cycle (day/night). The animals were fed standard granulated feed and had free access to water. The experimental group included 96 animals, the control group consisted of 24. The method of inhalation exposure to nitric oxide (NO) was used to reproduce pneumosclerosis. The concentration of NO in the air was 10 ppm for a long range. The exposure was carried out daily for 1 hour in a sealed chamber with a controlled gas composition. The exposure period was 6 days. This contributed to the induction of a chronic inflammatory process in the lung tissue, which is a key pathogenetic mechanism for the development of pneumosclerosis. After the end of the experiment, the animals were euthanized with the collection of lung tissue samples for histological and immunohistochemical examination.
3. Results and Discussion
- Based on the morphological parameters, the following morphometric data of the large intestine were obtained. In the morphological and morphometric study of the large intestine tissue obtained after dissection of white poor rats of the control group, the wall of the large intestine consists of 4 layers, the mucous layer is covered with a single-layer prismatic epithelium. In the field of view, high columnar epithelial cells, gocaloid cells and a large number of undifferentiated cells were observed. The mucous membrane is represented by a thin layer of connective tissue with a crypt, white fibrous tissue of the control group is on average 3.1-5.3 μm. The crypts were deep, slightly widened apical part, ix and medium in diameter, and the control squirrel crystallized 9.4-9.7 μm. Under the microscope, it was found that the crypts are in a state of weakly oxyphilic cytoplasm and in a state of gocaloid cells. The number of goblet cells in the crypts is 7–19 (Fig. 1).
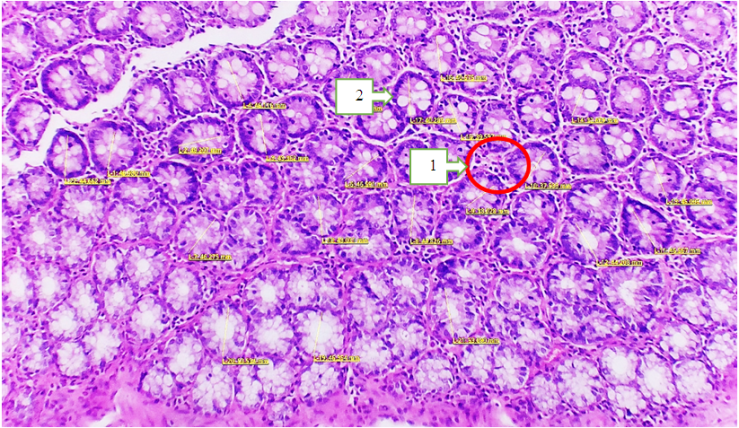 | Figure 1. Microscopic picture of the large intestine of white outbred rats of the main group. Hematoxylin and eosin staining. 40x20. 1-Cross-section of crypts; 2-goblet cells |
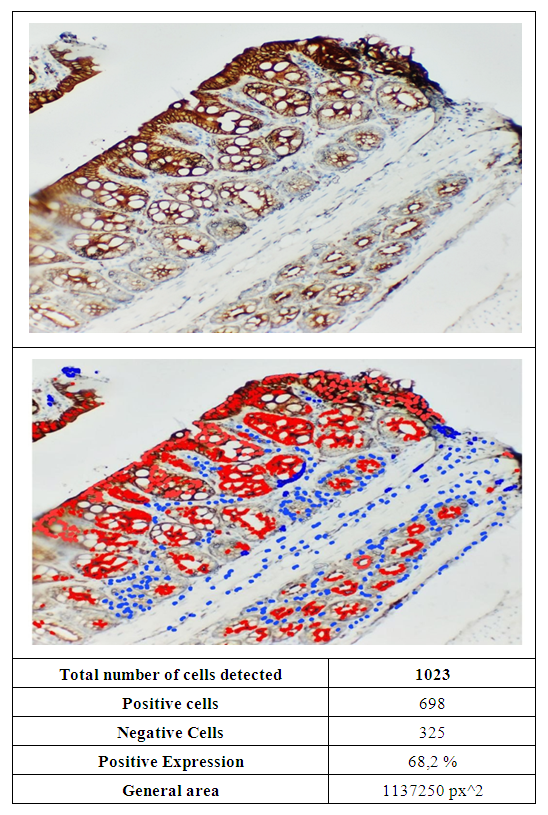
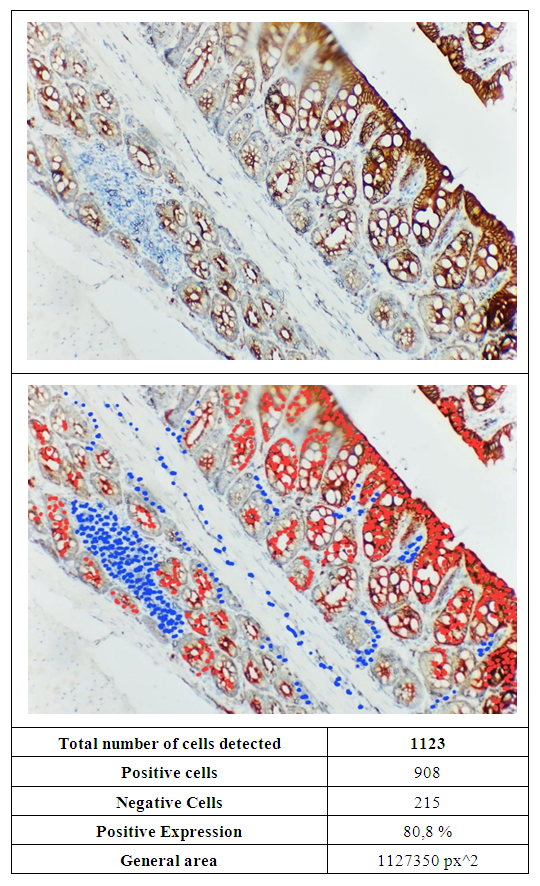 | Figure 2. In white rats of the experimental group, a high level of expression of the Ki-67 marker was observed in the tissue of the colon mucosa. The smear was stained with the chromogenic method |
4. Conclusions
- The study found high expression of the Ki-67 marker in the colonic mucosa and submucosa, especially in the crypt walls and goblet cells (86.6%, 80.8%, and 68.2%). This showed that cell proliferative activity increased in response to (NO2) nitrogen dioxide, a substance used to induce experimental pneumosclerosis. Changes in the morphological structure of the intestine against the background of pneumosclerosis may include mucosal atrophy, impaired epithelial regeneration and an increased inflammatory response, which is confirmed by immunohistochemical studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML