Zukhra Bakhtiyorovna Akhmedova1, Dilfuza Saburovna Matkarimova2, Kodirzhon Tukhtaboevich Boboev1
1Department of Molecular Medicine and Cellular Technologies, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
2Department of Hematology, Transfusiology and Laboratory Science, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Dilfuza Saburovna Matkarimova, Department of Hematology, Transfusiology and Laboratory Science, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Purpose of the study. To study the relationship of polymorphism of the proinflammatory cytokine IL1b (T-31C) gene with the development of aplastic anemia. Methods. The material for clinical and laboratory research in the work was AA patients (n=86) who sought diagnostic help and subsequent inpatient examination at the Republican Specialized Scientific and Practical Medical Center of Hematology (RNSPMH, Tashkent) in the period from 2019 to 2023. The age of patients with AA ranged from 18 to 79 years, with an average age of 40.8±1.8 years. The diagnosis was made based on clinical and laboratory data. Patients with AA (n=86), who made up the main group, were divided into three groups depending on the severity of the disease: mild, severe and super severe. To perform a molecular genetic analysis of immunomodulatory genes in AA patients and healthy patients, 4.0 ml venous blood samples were taken in tubes containing EDTA IL1b (T-31C), which were stored in freezers at a temperature of -80°C until the biomaterial was completely collected. After blood sampling, DNA from patients and healthy people was isolated using AmpliPrime RIBOT-prep reagents (Russia) on a NanoDrop 2000 spectrophotometer (NanoDropTechnologies, USA) at a wavelength of A260/280 nm, with DNA concentration determined in a ratio of 1.7/1.8. The polymorphic IL1b (T-31C) gene was detected by RT-PCR using a thermal cycler (Applied Biosystems 2720, USA; RotorGeneQ, QUAGEN Germany and Corbett Research - CG1-96, QUAGEN Germany). The amplification procedure was carried out by pre-denaturation of DNA at a temperature of 93°C for 1 minute; 35 denaturation cycles at a temperature of 93°C for 10 seconds; annealing of primers at a temperature of 64°C for 10 seconds; elongation process at a temperature of 72°C for 20 seconds; DNA synthesis at a temperature of 72°C for 1 minute; Separation of amplification products is carried out in a 3% agarose gel prepared on a TAE buffer by horizontal electrophoresis. To visualize the results of electrophoresis, a 1% solution of ethidium bromide is applied as a dye at the rate of 5 µl per 50 ml of molten gel. Fragments of the analyzed DNA appear as glowing orange-red stripes under UV radiation with a wavelength of 310 nm. LITEX test systems (Russia) were used to detect the studied genetic markers. The SNPs were tested for deviations from the Hardy-Weinberg equilibrium using the Chi-square test. The relative risk associated with alleles and genotypes was calculated as the odds ratio (OR) with a 95% confidence interval (CI), which were performed using the PC program “OpenEpi 2009, Version 2.3”. Conclusions. The results of the comparison of the established differences between polymorphic loci of the IL1b (T31C) gene in the studied groups, obtained on the basis of mathematical analysis, confirmed the above assumption. Thus, in the main group, compared with the control group, there was a statistically significant almost twofold increase in the frequency of the C mutant allele (39.5% vs. 25.5%; χ2=8.3; P=0.01; HR=1.2; DI: 0.78-1.95; OR=1.9; DI: 1.23-2.97), accompanied by a significant decrease protective effect of the main T alleles (60.5% vs. 74.5%; χ2=8.3; P=0.01; RR=0.8; DI: 0.53-1.23; OR=0.5; DI: 0.34-0.81) and the T/T genotype (37.2% vs. 58.2%; χ2=8.1; P=0.01; OR=0.6; DI: 0.34-1.22; OR=0.4; DI: 0.24-0.77).
Keywords:
IL1β (T-31C), Polymorphism, Allele, Genotype, Pancytopenia, Proinflammatory cytokine, Acquired aplastic anemia (AA)
Cite this paper: Zukhra Bakhtiyorovna Akhmedova, Dilfuza Saburovna Matkarimova, Kodirzhon Tukhtaboevich Boboev, Polymorphysis of the IL1β Gene (T-31C): Relationship to Aplastic Anemia, American Journal of Medicine and Medical Sciences, Vol. 15 No. 2, 2025, pp. 281-285. doi: 10.5923/j.ajmms.20251502.02.
1. Introduction
The pathogenesis of AA includes abnormal cellular immunity, gradual destruction of hematopoietic stem cells, and hematopoietic insufficiency, which can lead to a decrease in all blood cells [6,12]. Recent studies have shown that polymorphisms of cytokine genes increase the susceptibility of patients to the development of AA [4,10,13].IL-1b is the primary activator of early cytokines that facilitate the migration of leukocytes from blood vessels to tissues [5].The IL-1 family consists of three related genes, namely IL-1a, IL-1b, and IL-Ra, which encode the proinflammatory cytokines IL-1a and IL-1b and their IL-1R receptor [1,2]. IL-1 plays an important role in autoimmune diseases mediated by inflammation. It is known to be a potent pro-inflammatory cytokine with multiple biological effects, including cell proliferation, differentiation, and apoptosis [3,9,15].Various studies around the world have shown that genetic polymorphism plays a significant role in the development of AA [8,11,14]. The promoter sequence is a potential source of polymorphism affecting gene expression. Several single nucleotide polymorphisms (SNPs) have been reported in the regulatory region of cytokine genes, and some of them were associated with altered gene expression [1,7].
2. Main Body
2.1. The Purpose of Our Research
To study the relationship of polymorphism of the proinflammatory cytokine IL1b (T-31C) gene with the development of aplastic anemia.
2.2. Material and Methods of Study
The material for clinical and laboratory research in the work was AA patients (n=86) who sought diagnostic help and subsequent inpatient examination at the Republican Specialized Scientific and Practical Medical Center of Hematology (RNSPMH, Tashkent) in the period from 2019 to 2023. The age of patients with AA ranged from 18 to 79 years, with an average age of 40.8±1.8 years. The diagnosis was made based on clinical and laboratory data. Patients with AA (n=86), who made up the main group, were divided into three groups depending on the severity of the disease: mild, severe and super severe.To perform a molecular genetic analysis of immunomodulatory genes in AA patients and healthy patients, 4.0 ml venous blood samples were taken in tubes containing EDTA IL1b (T-31C), which were stored in freezers at a temperature of -80°C until the biomaterial was completely collected.After blood sampling, DNA from patients and healthy people was isolated using AmpliPrime RIBOT-prep reagents (Russia) on a NanoDrop 2000 spectrophotometer (NanoDropTechnologies, USA) at a wavelength of A260/280 nm, with DNA concentration determined in a ratio of 1.7/1.8. The polymorphic IL1b (T-31C) gene was detected by RT-PCR using a thermal cycler (Applied Biosystems 2720, USA; RotorGeneQ, QUAGEN Germany and Corbett Research - CG1-96, QUAGEN Germany).The amplification procedure was carried out by pre-denaturation of DNA at a temperature of 93°C for 1 minute; 35 denaturation cycles at a temperature of 93°C for 10 seconds; annealing of primers at a temperature of 64°C for 10 seconds; elongation process at a temperature of 72°C for 20 seconds; DNA synthesis at a temperature of 72°C for 1 minute; Separation of amplification products is carried out in a 3% agarose gel prepared on a TAE buffer by horizontal electrophoresis. To visualize the results of electrophoresis, a 1% solution of ethidium bromide is applied as a dye at the rate of 5 µl per 50 ml of molten gel. Fragments of the analyzed DNA appear as glowing orange-red stripes under UV radiation with a wavelength of 310 nm.LITEX test systems (Russia) were used to detect the studied genetic markers. The SNPs were tested for deviations from the Hardy-Weinberg equilibrium using the Chi-square test. The relative risk associated with alleles and genotypes was calculated as the odds ratio (OR) with a 95% confidence interval (CI), which were performed using the PC program “OpenEpi 2009, Version 2.3”.
2.3. Results of the Study
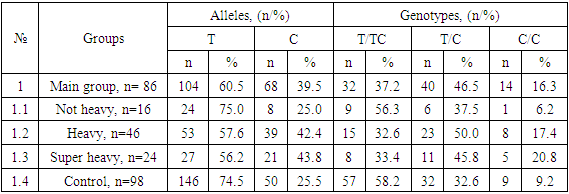
Studying the structural features of the IL1b polymorphic gene (T-31C) in a healthy control group (n=98), along with the dominance of the main T allele (74.5%) and the T/T genotype (58.2%), the recessive position of the mutant C allele (25.5%), the T/C heterozygote (32.6%) and mutant homozygote C/C (9.2%).However, in the main group (n=86) of AA patients, along with the dominance of the main T allele (60.5%) among the genotypic variants, the T/C heterozygote (46.5%) was dominant. In this group, the mutant C allele was detected with a frequency of 39.5%, the main homozygote – 37.2% and the mutant homozygote with a frequency of 16.3% (see Table 1).Table 1. Distribution of alleles and genotypes of IL1β (T-31C) rs1143627 polymorphism in groups of patients with aplastic anemia and healthy controls
 |
| |
|
Studying the structural features of the IL1b polymorphic gene (T-31C) in a healthy control group (n=98), along with the dominance of the main T allele (74.5%) and the T/T genotype (58.2%), the recessive position of the mutant C allele (25.5%), the T/C heterozygote (32.6%) and mutant homozygote C/C (9.2%).However, in the main group (n=86) of AA patients, along with the dominance of the main T allele (60.5%) among the genotypic variants, the T/C heterozygote (46.5%) was dominant. In this group, the mutant C allele was detected with a frequency of 39.5%, the main homozygote – 37.2% and the mutant homozygote with a frequency of 16.3% (see Table 1).The dynamics traced in the distribution of IL1b (T-31C) gene polymorphism in the healthy group was also typical for patients with mild AA (n=16), where the main T allele (75.0%) and T/T genotype (56.3%) were dominant, and the frequencies of the mutant C allele (25.0%), the T/C heterozygote (37.5%) and the C/C mutant homozygote (6.2%) were determined in smaller proportions.Along with this, the dominance of the main T allele in severe (57.6%) and super-severe (56.2%) forms of AA was accompanied by the dominance of the T/C heterozygote (50.0% and 45.8%) in both groups. Moreover, compared with the healthy group, in severe and super-severe forms of AA, the frequency of mutant C allele (42.4% and 43.8%) and C/C genotype (17.4% and 20.8%) increased markedly, respectively, with a decrease in the frequency of the main T/T homozygote to 32.6% and 33.4%, respectively (see Table 1).Analyzing the structure of the IL1b (T-31C) genetic polymorphism in the healthy group and among patients with AA, the dominance of the main allele T was found. However, in the main group, as well as in severe and super-severe AA, compared with the control and non-severe course, the dominance of the T/C heterozygote was established. Moreover, in the main group of AA patients, an increase in the frequency of mutant C alleles and C/C homozygotes was found due to patients with severe and super severe course. A noticeable increase in the frequencies of unfavorable alleles and genotypes may be related to their involvement in the development and severity of AA.The results of comparing the established differences between polymorphic loci of the IL1b (T-31C) gene in the studied groups, obtained on the basis of mathematical analyses, confirmed the above assumption. Thus, in the main group, compared with the control group, there was a statistically significant almost twofold increase in the frequency of the mutant C allele (39.5% vs. 25.5%; χ2=8.3; P=0.01; RR=1.2; CI: 0.78-1.95; OR=1.9; CI: 1.23-2.97), accompanied by a significant decrease in the protective effect of the main T alleles (60.5% vs. 74.5%; χ2=8.3; P=0.01; RR=0.8; DI: 0.53-1.23; OR=0.5; CI: 0.34-0.81) and T/T genotype (37.2% vs. 58.2%; χ2=8.1; P=0.01; RR=0.6; DI: 0.34-1.22; OR=0.4; DI: 0.24-0.77).In addition, an increase in the negative effect of the mutant C allele was combined with a tendency to increase the negative effect of the T/C heterozygote by 1.8 (46.5% vs. 32.7%; χ2 =3.7; P=0.1; RR=1.4; CI: 0.79-2.58; OR=1.8; CI: 0.99-3.25) and the C/C mutant homozygote by 1.9 times (16.3% vs. 9.2%; χ2 =2.1; P=0.2; RR=1.8; CI: 0.86-3.66; OR=1.9; DI: 0.8 - 4.65).Thus, the results of the study of the IL1b (T-31C) polymorphic gene in the main and control groups clearly prove the association of the studied polymorphism with an increased risk of AA. This is evidenced by the presence of a statistically significant increase in the frequency of the mutant C allele among patients compared with healthy ones by almost two times (χ2=8.3; P=0.01) and a tendency to increase the frequency of the T/C heterozygote by 1.8 (χ2 =3.7; P=0.1) and C/C by 1.9 times (χ2 =2.1; P=0.2), which was accompanied by a significant decrease in the positive effect of the main T alleles (χ2=8.3; P=0.01) and T/T homozygotes (χ2=8.1; P=0.01).A two-way comparison of the results between groups with mild AA and healthy ones showed no significantly significant differences in polymorphic loci for the IL1b (T-31C) gene. This was due to the very similar carriage rates of allelic and genotypic variants for the studied gene. For example, if the frequency of the mutant A allele in mild AA was 25.0%, then in the healthy group it was 25.5% (χ2<3.84; P=0.98; RR=1.0; CI: 0.79-1.26; OR=1.0; CI: 0.41-2.31), then between the frequencies of the main G/G genotype (56.3% vs. 58.2%; χ2<3.84; P=0.9; RR=1.0; CI: 0.16-5.81; OR=0.9; CI: 0.32-2.69), G/A heterozygotes (37.5% vs. 32.7%; χ2=0.1; P=0.8; RR=1.1; DI: 0.18-7.16; OR=1.2; CI: 0.41 - 3.7) and mutant homozygotes (6.3% vs. 9.2%; χ2=0.1; P=0.8; RR=0.7; DI: 0.02-29.2; OR=0.7; CI: 0.08-5.51) also, no pronounced differences were found.Differences in statistically significant levels in the frequencies of the mutant C allele were found between polymorphic loci of the IL1b (T-31C) gene in the group with severe AA compared with the control group, the proportion of which was 2.1 times higher among patients in this group (42.4% vs. 25.5%; χ2=8.4; P=0.01; RR=1.3; DI: 0.87-1.92; OR=2.1; CI: 1.28-3.61) and T/C heterozygotes, which were also 2.1 times more common among patients (50.0% vs. 32.7%; χ2 =4.0; P=0.05; RR=1.5; CI: 0.61-3.85; OR=2.1; DI: 1.01 - 4.2). All this was accompanied by a significant decrease in the protective effect of the main T alleles (57.6% vs. 74.5%; χ2=8.4; P=0.01; RR=0.8; DI: 0.41-1.47; OR=0.5; DI: 0.28-0.78) and T/T genotype (32.6% vs. 58.2%; χ2=8.2; P=0.01; RR=0.6; DI: 0.2 - 1.56; OR=0.3; DI: 0.17-0.72) among the group of patients with AA, creating prerequisites for the development of a severe form of the disease.The frequency of C/C mutant homozygotes showed a slight tendency to increase among patients by 2.1 times (17.4% vs. 9.2%; χ2 =2.0; P=0.2; RR=1.9; CI: 0.62-5.79; OR=2.1; DI: 0.76-5.72).Thus, the results of the comparison between groups of patients with severe AA and healthy ones prove the presence of a significant association of the IL1b (T-31C) polymorphic gene with the risk of severe AA. The mutant A allele (χ2=8.4; P=0.01) and the T/C heterozygote (χ2 =4.0; P=0.05) significantly increased the risk of this form of AA by 2.1 times.According to polymorphic loci of the IL1b (T-31C) gene, differences between the groups with the super-severe form of AA and the control group were also significant in the frequency of the mutant C allele (43.8% vs. 25.5%; χ2=6.2; P=0.03; RR=1.3; CI: 0.96-1.83; OR=2.13 CI: 1.19-4.33). Moreover, the development of the superheavy course of AA was facilitated by a significant decrease in the frequencies of the protective main T alleles (56.3% vs. 74.5%; χ2=6.2; P=0.03; RR=0.8; DI: 0.28-2.01; OR=0.4; DI: 0.23-0.84) and T/T genotype (33.3% vs. 58.2%; χ2=4.8; P=0.05; RR=0.6; DI: 0.13-2.59; OR=0.4; CI: 0.14 - 0.9) among the group of AA patients, creating the prerequisites for the development of a severe form of the disease.There were no significant differences in the frequency of T/C heterozygotes between the studied groups (45.8% vs. 32.7%; χ2 =1.5; P=0.3; RR=1.4; CI: 0.35-5.66; OR=1.7; CI: 0.71 - 4.3). However, the frequency of C/C mutant homozygotes, although with a weak trend, still increased the risk of super-severe AA by 2.6 times (20.8% vs. 9.2%; χ2 =2.6; P=0.2; RR=2.3; DI: 0.46-11.1; OR=2.6; DI: 0.81-8.37).Thus, the above results prove the presence of a significant association between polymorphic loci of the IL1b (T-31C) gene and the risk of developing the super severe form of AA.In particular, a significant risk is associated with the carriage of the mutant C allele, which increases the risk of superheavy AA by 2.3 times (χ2=6.2; P=0.03).Assessing the significance of differences in polymorphic loci of the IL1b (T-31C) gene in the group of patients with mild AA compared with severe and super-severe forms of AA, there was a tendency for the frequencies of functionally favorable main T alleles to increase in 2.2 (75.0% vs. 57.6%; χ2=3.1; P=0.1; RR=1.3; DI: 0.32 - 5.27; OR=2.2; CI: 0.91 - 5.37) and 2.3 times (75.0% vs. 56.3%; χ2=2.9; P=0.1; RR=1.3; CI: 0.37 – 4.84; OR=2.2; CI: 0.88-6.17), respectively, and the T/T genotype by 2.7 times (56.3% vs. 32.6%; χ2=2.8; P=0.1; RR=1.7; CI: 0.33 - 9.04; OR=2.7; CI: 0.85 - 8.35) and 2.1 times (56.3% vs. 33.3%; χ2=2.1; P=0.2; RR=1.7; CI: 0.38 – 7.53; OR=2.6; DI: 0.71 – 9.33), respectively, having a protective effect on the severity of the AA course.In the group with a mild form of AA, compared with severe and super-severe forms of the disease, the carriers had unfavorable alleles (C: 25.0% vs. 42.4%; χ2=3.1; P=0.1; RR=0.8; DI: 0.52-1.13; OR=0.5; DI: 0.19-1.1 and 25.0% vs. 43.8%; χ2=2.9; P=0.1; RR=0.8; DI: 0.38-1.47; OR=0.4; CI: 0.16-1.13) and genotypic variants (T/S: 37.5% vs. 50.0%; χ2=0.7; P=0.4; RR=0.8; DI: 0.13-4.21; OR=0.6; DI: 0.19-1.91 and 37.5% vs. 45.8%; χ2=0.3; P=0.7; RR=0.8; DI: 0.17-3.88; OR=0.7; DI: 0.2-2.58; C/C: 6.3% vs. 17.4%; χ2=1.3; P=0.9; RR=0.4; DI: 0.01-14.79; OR=0.3; DI: 0.04-2.51 and 6.3% vs. 20.8%; χ2=1.6; P=0.3; RR=0.3; DI: 0.01-10.8; OR=0.3; CI: 0.03-2.12), there were no statistically significant differences in the studied gene.At that time, there were practically no statistically significant differences in polymorphic loci of the IL1b (T-31C) gene between the groups of patients with severe and super-severe forms of AA (C: 42.4% vs. 43.8%; χ2<3.84; P=0.9; RR=1.0; Di: 0.4-2.41; HR=0.9; Di: 0.47-1.91 t/s: 50.0% vs. 45.8%; χ2<3.84; P=0.98; RR=1.0; Di: 0.48-1.99; or=1.0; Di: 0.34-2.76 and c/s: 17.4% vs. 20.8%; χ2=0.1; P=0.8; RR=0.8; Di: 0.33-2.09; HR=0.8; Di 0.23-2.78).
3. Conclusions
The results of comparative analyses between polymorphic loci of the IL1b (T-31C,) gene and the risk of AA development proved the presence of a statistically significant relationship. In particular, the risk of developing AA was significantly increased among carriers of the mutant C allele compared with healthy ones by almost two times. When carrying a T/C heterozygote and a mutant C/C homozygote, there was a tendency to increase the risk of AA by 1.8 and 1.9 times.In addition, there was a significant association between the mutant C allele and the C/T heterozygote with a 2.1-fold increased risk of severe AA, as well as a significant association between the mutant C allele and a 2.3- and 2.6-fold increased risk of this form of AA when carrying the mutant C/T homozygote, respectively.
References
| [1] | Banday M. Z. et al. Interleukin-10− 592C/A, but not− 1082A/G promoter single nucleotide polymorphism, is associated with a decreased risk of colorectal cancer in an ethnic Kashmiri population: a case–control study // European Journal of Cancer Prevention. – 2017. – Т. 26. – №. 6. – С. 476-490. |
| [2] | Borthwick L. A. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung // Seminars in immunopathology. – Springer Berlin Heidelberg, 2016. – Т. 38. – С. 517-534. |
| [3] | Chen L. et al. Inflammatory responses and inflammation-associated diseases in organs // Oncotarget. – 2018. – Т. 9. – №. 6. – С. 7204. |
| [4] | Deng S. et al. The relationship between interferon-gamma (INF-γ) single nucleotide polymorphism+ 874 (T/A) and occurrence risk of aplastic anemia: a meta-analysis // Hematology. – 2020. – Т. 25. – №. 1. – С. 85-90. |
| [5] | Hemmati S., Haque T., Gritsman K. Inflammatory signaling pathways in preleukemic and leukemic stem cells // Frontiers in oncology. – 2017. – Т. 7. – С. 265. |
| [6] | Karantanos T., DeZern A. E. Biology and clinical management of hypoplastic MDS: MDS as a bone marrow failure syndrome // Best Practice & Research Clinical Haematology. – 2021. – Т. 34. – №. 2. – С. 101280. |
| [7] | Liang X. et al. Polymorphisms of the TGF-β1 gene and the risk of acquired aplastic anemia in a Chinese population // Annals of Hematology. – 2017. – Т. 96. – С. 339-344. |
| [8] | Luzzatto L, Risitano AM. Advances in understanding the pathogenesis of acquired aplastic anaemia. Br J Haematol. 2018; 182: 758–76. doi: 10.1111/bjh.15443. |
| [9] | Netea M. G. et al. A guiding map for inflammation //Nature immunology. – 2017. – Т. 18. – №. 8. – С. 826-831. |
| [10] | Shukla S. et al. Association of interleukin-1β-31C/T, -511T/C and-3954C/T single nucleotide polymorphism and their blood plasma level in acquired aplastic anemia // Indian Journal of Hematology and Blood Transfusion. – 2021. – Т. 37. – С. 210-219. |
| [11] | Wang X. A. et al. Mesenchymal stem cells in acquired aplastic Anemia: the Spectrum from Basic to Clinical Utility // International Journal of Molecular Sciences. – 2023. – Т. 24. – №. 5. – С. 4464. |
| [12] | Young N. S. Aplastic anemia // New England Journal of Medicine. – 2018. – Т. 379. – №. 17. – С. 1643-1656. |
| [13] | Zayed R. A., Abdel-Hamid S. M., El-Lithy H. The association of cytokine genes polymorphisms and susceptibility to aplastic anemia in Egyptian patients // Hematology. – 2016. – Т. 21. – №. 2. – С. 106-112. |
| [14] | Zhang X. et al. Interleukin-2 and Interleukin-8 gene polymorphisms and acquired aplastic anemia risk in a Chinese population // Cellular Physiology and Biochemistry. – 2017. – Т. 41. – №. 3. – С. 1199-1207. |
| [15] | Zhong J., Shi G. Regulation of inflammation in chronic disease // Frontiers in Immunology. – 2019. – Т. 10. – С. 737. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML