-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(1): 255-258
doi:10.5923/j.ajmms.20251501.50
Received: Dec. 23, 2024; Accepted: Jan. 17, 2025; Published: Feb. 3, 2025

The Significance of Annexine A1 Level Changes in the Course of Axial Spondylarthritis
M. Sh. Karimov, D. N. Ikramova, A. A. Eshmurzaeva, Kh. S. Akhmedov
Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article presents the results of a study on the relationship between the level of anneksin A1 (ANXA1) in the blood of 62 patients with different clinical stages of axial spondylitis (AS) and the degree of disease activation. According to the results obtained, against the backdrop of a decrease in ANXA1 levels in the blood serum of patients with AS, exacerbation of the disease is observed.
Keywords: Axial spondylarthritis, Activation, Annexin A1
Cite this paper: M. Sh. Karimov, D. N. Ikramova, A. A. Eshmurzaeva, Kh. S. Akhmedov, The Significance of Annexine A1 Level Changes in the Course of Axial Spondylarthritis, American Journal of Medicine and Medical Sciences, Vol. 15 No. 1, 2025, pp. 255-258. doi: 10.5923/j.ajmms.20251501.50.
Article Outline
1. Introduction
- Currently, understanding of the mechanism of corresponding structural changes in the structure of the spine in axial spondylitis (AS) has been formed, and according to modern understanding [17], not only autoimmune, but also hyperinflammatory processes occupy a special place. Furthermore, considering the association of the disease with a person's primary histological adaptation complex of type I (AGM-I) based on the general pathogenesis, AS is considered to belong to the category of "AGM-I-associated diseases" [15]. The origin of AS is associated with the immune system response and the expression of pro-inflammatory cytokines, particularly TNF-α, IL-17, and IL-23, whose hyperscretion is observed based on a specific genetic predisposition of T-cell immunity. Increased levels of these pro-inflammatory cytokines in the corresponding tissues contribute to the development of an inflammatory process associated with increased prostaglandin E2 (PgE2) and type 2 cycloxygenase (COH-2) [12].According to the literature [11], the activation of the pathological process is accompanied by structural changes in the spinal tissues, which arise due to cascade changes. These changes, initially occurring at the molecular stage, subsequently lead to pronounced anatomical and physiological disorders in the spine and exacerbation of AS [13]. Increased pain in the spine in this disease corresponds to the radiological stage of the disease, and an increase in the number of visits to the doctor during this period is observed during the period of exacerbation of the disease and the development of irreversible ankylosing spinal disorders [5]. Therefore, the isolation of biomarkers that allow for early diagnosis of AS and assessment of structural changes in the spine is of scientific interest. In the literature of subsequent years, patients with HLA-B27 develop specific cytotoxic T-lymphocytes with a TRBV9 segment on the receptor, which are also called "autoreactive T-lymphocytes" [16].This segment is involved in the recognition of foreign antigens. When the immune system is disrupted, cytotoxic T-lymphocytes activate and attack healthy cells. As a result, the spine, sacro-ilial junction, and other organ tissues are inflamed and AS develops.In recent years, scientists' focus on the protein Anneksin A1 (ANXA1) has been linked to its role in the immune system [4]. Because it is synthesized by immune system cells under the influence of glucocorticoids [3]. Based on this, ANHA1 indirectly exhibits immunosuppressive, anti-inflammatory, and anti-allergic properties. However, it suppresses the activity of phospholipase A2, contributing to a decrease in prostaglandin and leukotriene production. Furthermore, ANXA1 enhances the suppression of prostaglandin biosynthesis by inhibiting types 1 and 2 cyclooxygenase [14].Along with this, ANXA1 binds to specific receptors on the leukocyte membrane and attempts to inhibit various manifestations of its activity. Consequently, ANXA1 suppresses processes such as the adhesion ability of epithelial cells, leukocyte migration from blood to target tissue, chemotaxis, phagocytosis, and oxidative metabolism. ANXA1 plays an important role in inhibiting the production of various pro-inflammatory mediators, including lysosomal enzymes, cytokines, and plasminogen activators, by neutrophils, macrophages, and mast cells.It is well-known that the clinical course and joint syndrome of many rheumatic diseases largely depend on morphological changes, the inflammatory process, and geographical factors [2]. Certainly, the varying development of rheumatic diseases and their distinctive progression patterns lead to specific changes in the joints [9]. Among these conditions, AS is characterized by diverse progression patterns, manifesting through various clinical and radiological changes as well as functional states. This, in turn, results in the exacerbation of structural disorders in the spine, ultimately leading to a deterioration in the patients' quality of life [1].Therefore, taking into account the role of ANXA1 in the inflammatory process, the proposed method aims to establish its role in the early detection of changes in the developing tissues of the spine in AS, as well as to determine its prognostic significance in structural changes.The aim of the study is to determine the change in ANXA1 against the background of disease activation in patients with AS.
2. Research Materials and Methods
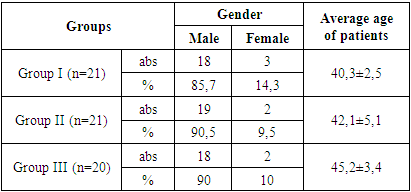
- This study involved 62 patients with various clinical stages of ankylosing spondylitis (AS), with an average age of 43.2±5.3 years and a mean disease duration of 2.4±1.4 years. The control group consisted of 10 healthy individuals (average age 41.5±4.4 years) approximately matched to the selected patients by age and sex. For the purpose of the study, patients with AS were divided into 3 groups (Table 1): Group I (n=21) included patients in the non-radiographic stage of AS; Group II (n=21) included patients in the established stage of AS; and Group III (n=20) included patients in the late stage of AS. Group II (n=21) - expressed stage of AS; Group III (n=20) included patients with late stages of AS.
|
3. The Results Obtained and Their Discussion
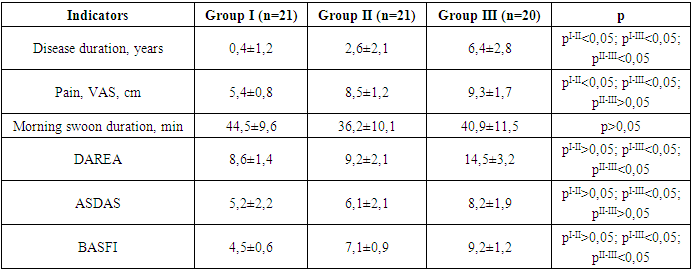
- The majority of patients participating in this study, 88.7%, were men. The duration of the disease was 2.5±1.1 years, and according to medical history, the average age of patients at the time of the onset of the first symptoms was 40.2±2.6 years. The average period before the diagnosis was established with the onset of the first symptoms was 12.5 months.
|
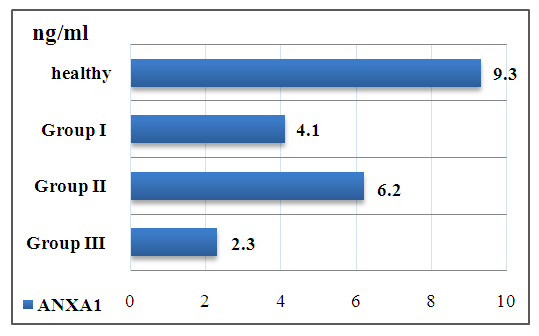 | Figure 1. Changes in ANXA1 levels in groups |
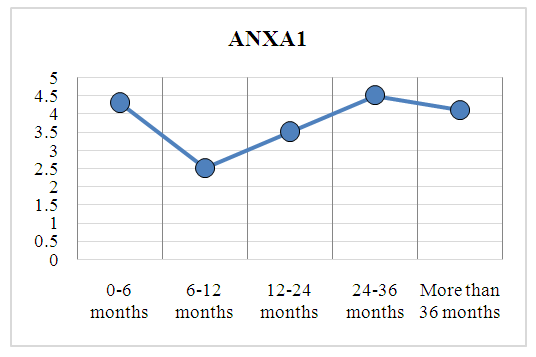 | Figure 2. Changes in ANXA1 levels depending on the duration of AS |
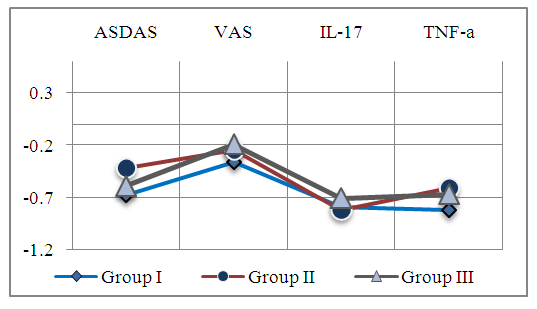 | Figure 3. The correlation between ANXA1 and the inflammatory process |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML