-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(1): 219-227
doi:10.5923/j.ajmms.20251501.43
Received: Dec. 27, 2024; Accepted: Jan. 19, 2025; Published: Jan. 27, 2025

Evaluation of Renal Hemodynamics and the Effect of Empagliflozin on It in Chronic Heart Failure of Various Etiologies
Tosheva Khakima Bekmurodovna1, Khotamova Raykhon Sulaymonovna2, Gadaeva Nilufar Abdigaffarovna3
1Associate Professor, PhD, Department of Propaedeutics of Internal Diseases, Bukhara State Medical University named after Abu Ali ibn Sino, Bukhara, Uzbekistan
2Assistant, Department of Internal Medicine, Bukhara State Medical University named after Abu Ali Ibn Sina, Bukhara, Uzbekistan
3Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Khotamova Raykhon Sulaymonovna, Assistant, Department of Internal Medicine, Bukhara State Medical University named after Abu Ali Ibn Sina, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The analysis showed that the resistance index is high in rheumatic heart defects. After standard treatment with sodium glucose cotransporter 2 inhibitors, the resistance index decreased in patients and led to positive changes in kidney function. Correlations between the resistance index and a number of renal markers were also studied. In chronic heart failure developed on the basis of rheumatic heart defects, inflammatory processes are long-term and latent. The article compares renal hemodynamic parameters before and after treatment in patients with chronic heart failure developed on the basis of rheumatic heart defects and ischemic heart disease.
Keywords: Chronic heart failure (CHF), Coronary heart disease (CHD), KIM 1 (Kidney Injury Molecula 1), Renal hemodynamic, Cystatin C, Tumor necrosis factor alpha
Cite this paper: Tosheva Khakima Bekmurodovna, Khotamova Raykhon Sulaymonovna, Gadaeva Nilufar Abdigaffarovna, Evaluation of Renal Hemodynamics and the Effect of Empagliflozin on It in Chronic Heart Failure of Various Etiologies, American Journal of Medicine and Medical Sciences, Vol. 15 No. 1, 2025, pp. 219-227. doi: 10.5923/j.ajmms.20251501.43.
1. Introduction
- The cardiovascular continuum is a chain that links interrelated changes. This chain consists of the occurrence and development of endothelial dysfunction caused by the combined effects of risk factors such as arterial hypertension, diabetes mellitus, obesity, dyslipidemia, tobacco smoking, etc. atherosclerosis, left ventricular hypertrophy, coronary heart disease, including myocardial infarction, as well as heart failure and death [15]. The procedure is accompanied by damage to the kidneys, as well as a number of other organs, and the final part of this chain is chronic heart failure (CHF).CHF is considered a syndrome of various etiologies, multifactorial, indicating a decrease in cardiac function, in which the blood supply to the organs is disrupted in accordance with the body's needs. Although this severe complication is most often observed in hypertension and coronary heart disease (CHD), among its many causes is chronic rheumatic heart disease. It should be noted that among the factors influencing the outcome of CHF, developed aortic stenosis due to rheumatism, as well as mitral insufficiency, as shown, occupy one of the leading places.The pathological interaction of these substances on each other in the close relationship between the heart and kidneys leads early to negative consequences in patients with chronic heart failure. Since these two cases have become increasingly common in the population in the last decade, a double epidemic of heart and kidney failure has been mentioned in many cases [11]. In addition, the simultaneous occurrence of these clinical conditions in most patients has led to the fact that pronounced cardiorenal syndrome has entered practice [19,20]. The phrase expresses the presence of dysfunction (insufficiency) in the work of the heart and kidneys in patients simultaneously [1,7].Indeed, a meta-analysis (n=18634) by K. Dattan et al. showed that renal impairment in chronic heart failure creates a 61% risk of death, increasing rehospitalization by 30% over 2-6 months of follow-up [3,4]. Similar results were obtained by MaAlister et al. They noted that approximately 50% of patients have a SCF of less than 60 ml per 1.73 m² of body surface (per minute) [10]. According to a study by A. Kotgen, there were 18 cases of patients with SCF less than 60 ml per minute per 1.73 m² of body surface per 1000 people per year [8]. In male patients with CHF, blood creatinine levels above 132.6 μmol/l, uncontrolled systolic (above 200 mmHg) blood pressure, heart rate above 100 beats per minute, wheezing outside the basal part of the lungs, comparisons, patient age and renal dysfunction in diabetes mellitus are predictors [2,5,6,9].Hypotension and hypoperfusion observed in chronic heart failure lead to compensatory activation of the renin-angiotensin-aldosterone and sympathetic systems.By activating this system, angiotensin II stimulates the sympathetic nervous system, which innervates afferent and efferent blood vessels. Its activation causes contraction of both vessels, which in turn causes a decrease in renal blood flow and glomerular filtration rate [12,13]. Angiotensin II also causes an increase in arginine vasopressin levels, which in turn contributes to the development of chronic kidney disease [14].Some authors have shown a correlation between the state of renal blood flow and the outcome of chronic heart failure. PV. Ennezat et al. were the first to demonstrate a negative prognostic value of the renal vascular resistance index in patients with chronic heart failure. This was confirmed in studies by a number of other authors [16]. In addition, the prognostic significance of renal volume flow indices in chronic heart failure has been demonstrated in the Russian population [17,18].However, the influence of the above factors on cardiorenal relationships has been studied in most cases of chronic heart failure developing on the basis of ischemic heart disease and arterial hypertension. Data on renal dysfunction, including its hemodynamics, in chronic heart failure caused by chronic rheumatic heart disease have not been sufficiently described in the literature. From this point of view, their study is one of the most pressing problems in medicine.
2. Materials and Methods
- This study was conducted in 2022 and 2023 in patients with CHF due to rheumatic heart disease who were treated at the clinic of the Bukhara State Medical Institute and the regional multidisciplinary medical center. Among them, the observation included patients whose serum creatinine level exceeded the norm over the past 3 months and whose SCF calculated with its help per 1.73 m2 of body surface area was from 60 to 90 per minute. To implement the solution of the set research objectives, the following was carried out.The study involved 100 patients with chronic heart failure who were treated in a hospital setting and then were under observation. The first group consisted of 60 patients with chronic heart failure due to rheumatic heart disease, and the second group consisted of 40 patients with CHF due to ischemic heart disease and hypertension. The control group included 20 healthy individuals. The average age of the first group was 46.1±1.3 years, among them there were 22 men (36.6%) and 38 women (63.4%). The average age of the second group was 56.1±1.5 years, it included 25 (62.5%) men and 15 (37.5%) women. The average age of the control group was 41.4±1.2 years, 10 (50%) men and 10 (50%) women.Patients included in the study underwent clinical, laboratory and functional examinations. Diagnosis of chronic heart failure and its functional classes is based on patient complaints, anamnesis, objective examination data and laboratory and instrumental studies in accordance with the "Recommendations for the diagnosis and treatment of acute and chronic heart failure". "Refusal" updated in 2023 by the criteria of the European Society of Cardiology and the New York Heart Association (New York Heart Association, 1964). A thorough anamnesis was collected from patients, special attention was paid to previous rheumatism, echocardiographic changes confirming the presence of heart defects, and risk factors for the development of chronic heart failure.Based on the stated goals and objectives, the results before and after various complex standard treatment methods were studied and analyzed, taking into account the functional class of patients with chronic heart failure, as discussed above. All subjects included in the study underwent a general clinical examination before treatment and 6 months after it. Also, based on the stated goal, KIM 1 (Kidney Injury Molecula 1), α-tumor necrosis factor, interleukin-6, creatinine and cystatin-C were determined in the blood for the purpose of comparative assessment of renal dysfunction. All patients underwent Doppler ultrasonography of the renal vessels to assess renal hemodynamics. All study participants were regularly monitored for 6 months and underwent repeated laboratory and instrumental examinations.All patients were prescribed standard therapy for chronic heart failure: ACE inhibitors or angiotensin receptor antagonists, β-blockers, mineralocorticoid receptor antagonists, and, depending on the indications, diuretics, cardiac glycosides and antiarrhythmic drugs. The first group, in addition to the standard treatment complex mentioned above, was also recommended empagliflozin, an inhibitor of the sodium-glucose cotransporter type 2.Doppler study of renal vessels.Doppler studies were performed before and after treatment to determine the resistance of the renal arteries and blood flow velocity in the observed patients. The study was conducted in the Bukhara Regional Hospital using a diagnostic device with color Doppler mapping Sonoscape S20. The method of spectral analysis of blood flow velocity and vascular resistance in the main, segmental and intersegmental renal veins was used. The following parameters were determined at the confluence of the right and left renal arteries:- PSV - systolic blood flow velocity, cm/sec;- EDV - diastolic blood flow velocity, cm/sec;In intrarenal arteries:- segmental (SA) – V max, Vd;- intersegmental (MA) – V max, Vd;- main (RA) – V max, Vd.Renal vascular resistance (RI) was calculated to assess renal vascular resistance. The threshold value of the interlobar artery resistance index was 0.64. Resistance index: determined using peak systolic velocity of arterial blood flow (PSV) - end diastolic velocity (EDV) / peak systolic velocity of arterial blood flow (PSV). According to the literature, this index is the main indicator determining changes in renal hemodynamics.
3. Result and Discussion
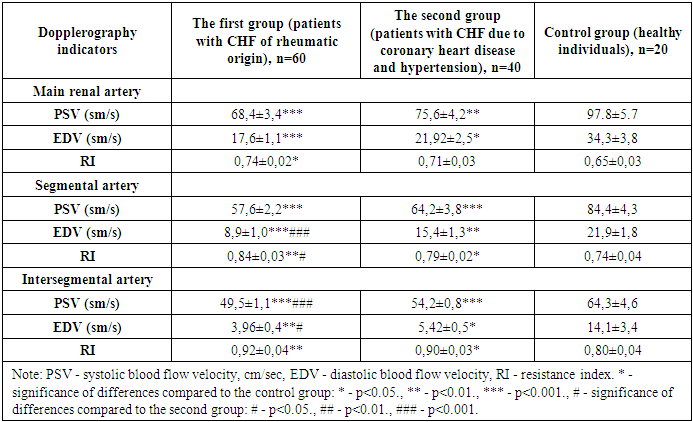
- In patients included in the study, the results of intrarenal hemodynamics obtained before and after treatment were compared. In addition, a number of other factors affecting intrarenal hemodynamics were studied.Renal hemodynamics were assessed based on changes in hemodynamics in its main, segmental and intersegmental arteries. It is worth noting that both groups had an equal number of patients with chronic heart failure of functional classes II and III. The results obtained are presented in Table 1.
|
|
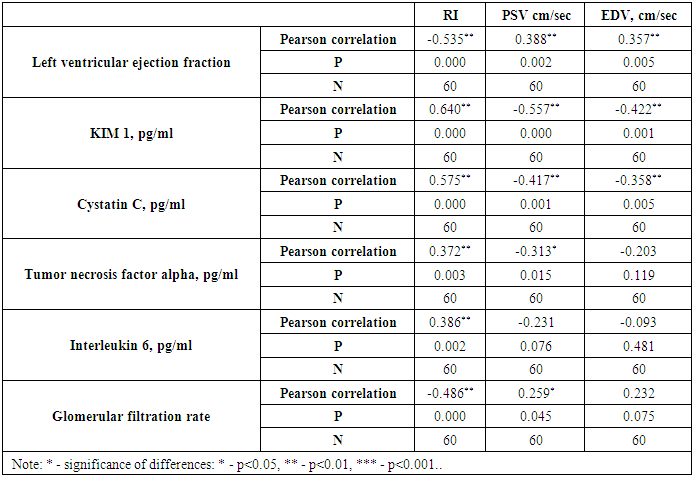
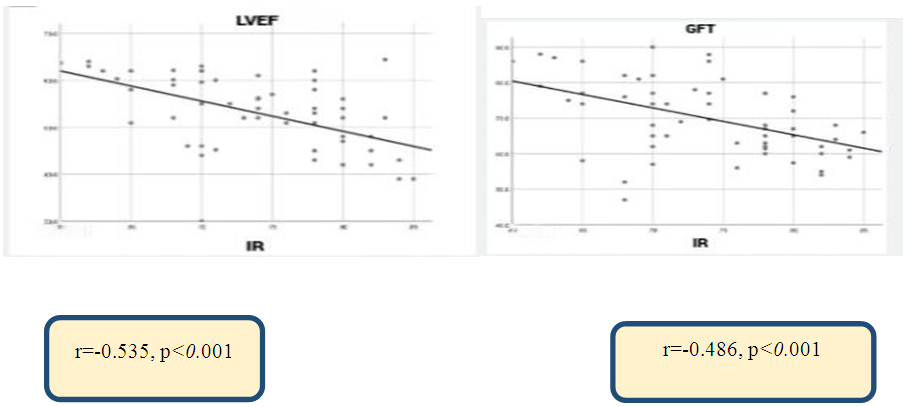 | Figure 1. Correlation between renal vascular resistance (RI) index with left ventricular ejection fraction and glomerular filtration rate in chronic heart failure caused by rheumatic heart disease |
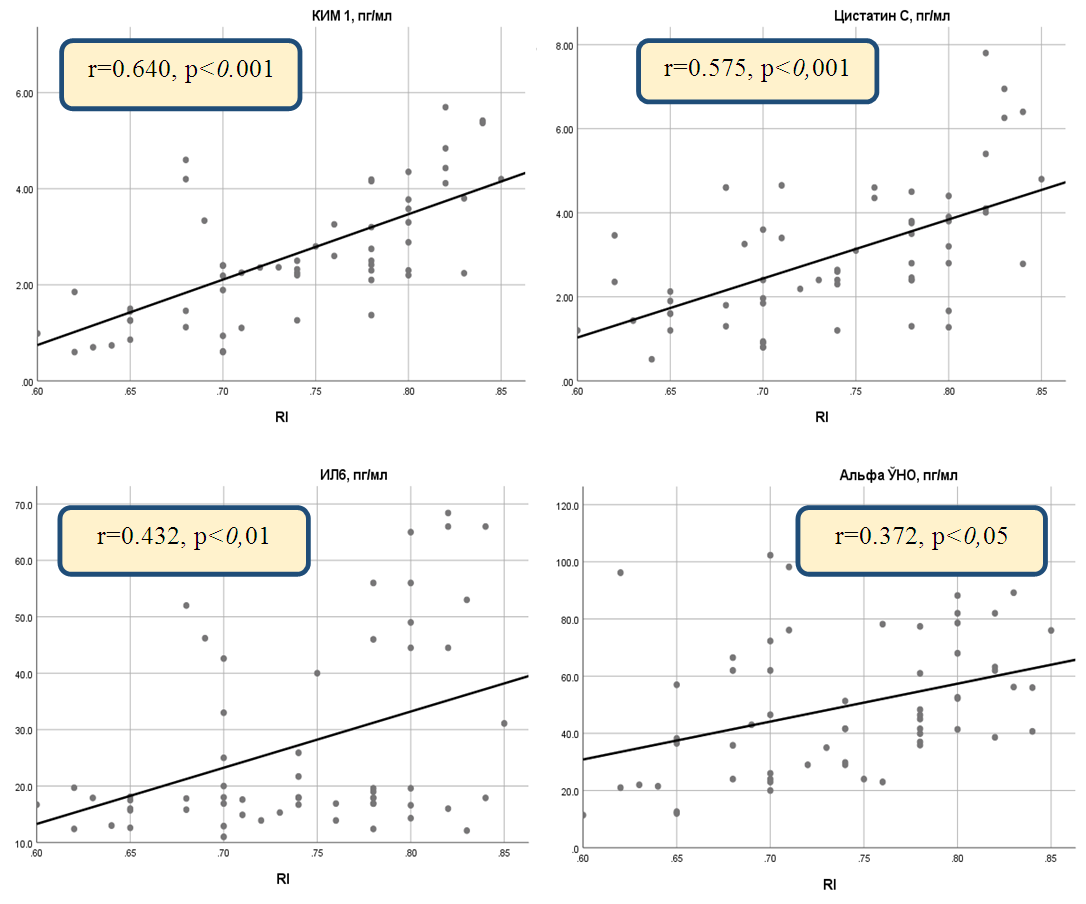 | Figure 2. Correlation between resistance index (RI) and inflammatory cytokines in chronic heart failure caused by rheumatic heart disease |
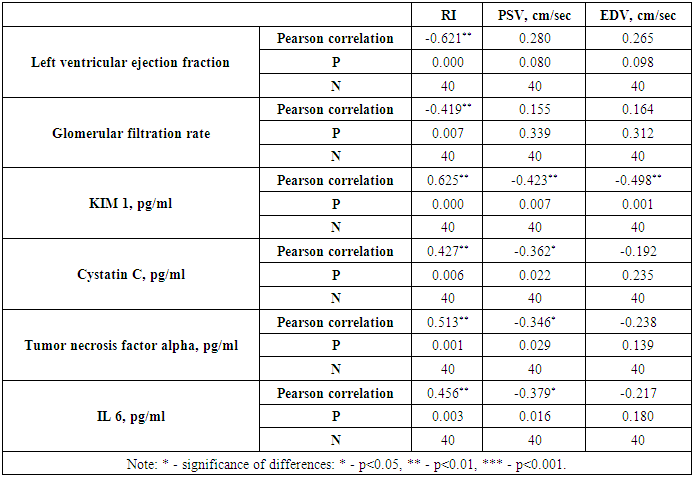
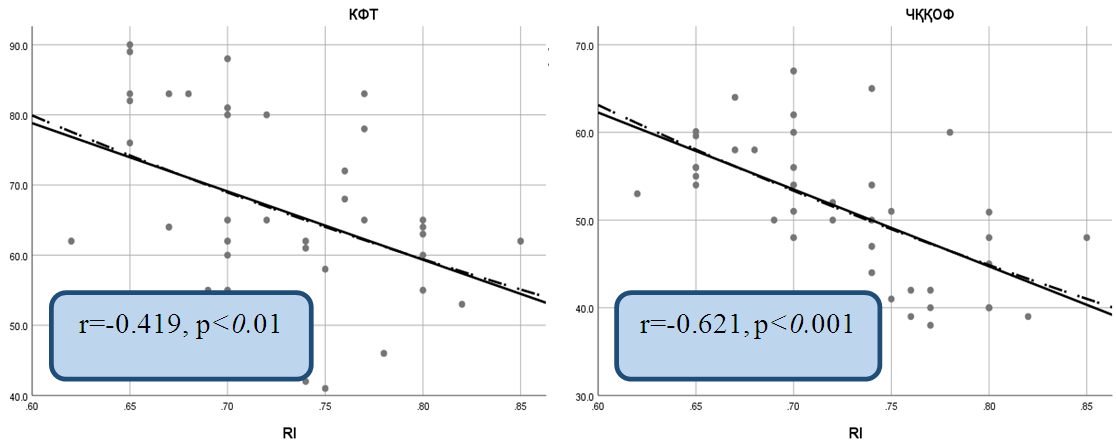 | Figure 3. Correlation between the resistance index (RI) with left ventricular ejection fraction and estimated glomerular filtration rate in chronic heart failure due to coronary heart disease |
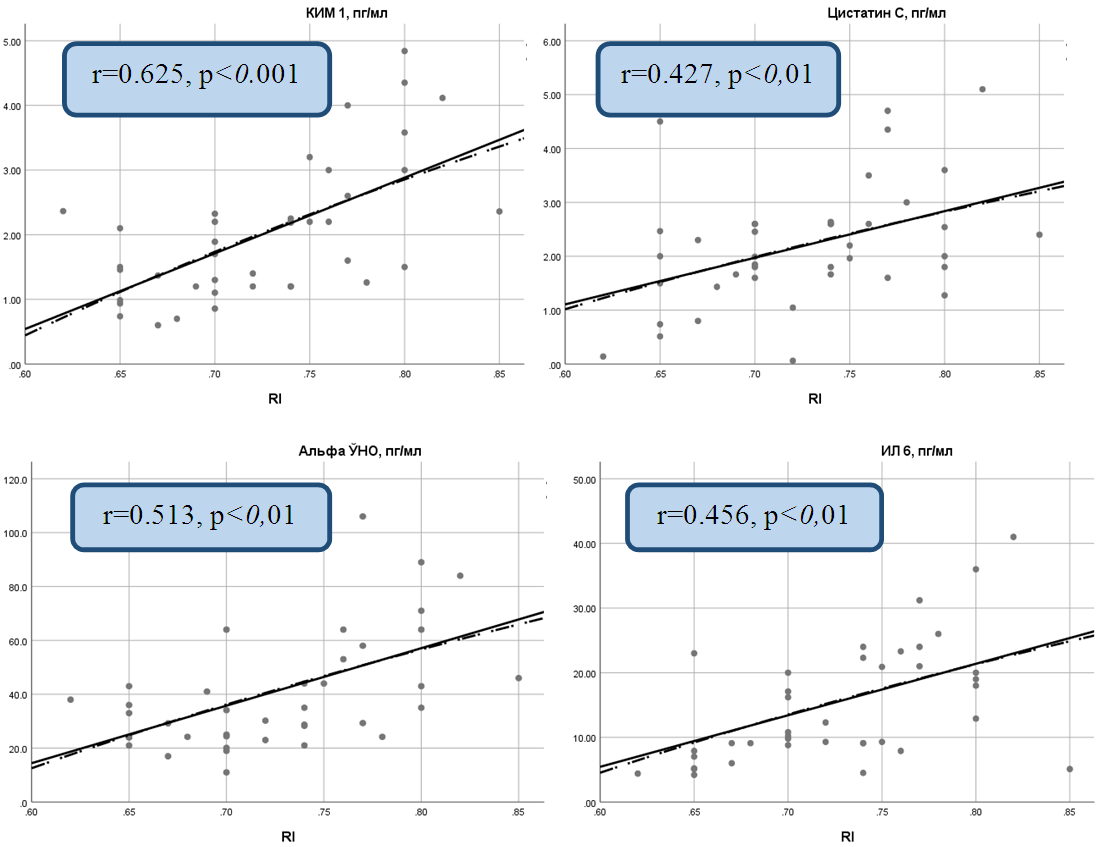 | Figure 4. Correlation between resistance index (RI) and inflammatory cytokines in chronic heart failure caused by coronary artery disease |
|
4. Conclusions
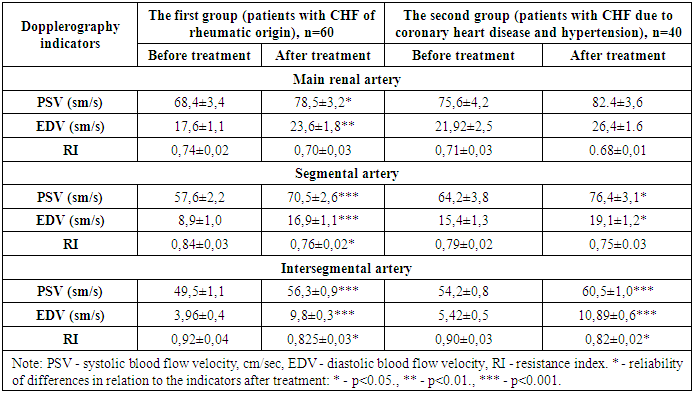
- The resistance index is one of the important Doppler indices in assessing renal hemodynamics in patients with chronic heart failure. In most cases of chronic heart failure, there is an increase in systemic and renal vascular resistance due to activation of the renin-angiotensin-aldosterone and sympathetic nervous systems. These changes lead to vasoconstriction and increased renal vascular resistance. In addition, in chronic heart failure, increased central venous pressure also leads to congestion in the renal veins. This, in turn, affects the pressure gradient in the renal tubules, causing an increase in the resistance index. These pathophysiological changes, which are also observed in chronic heart failure, usually lead to an increase in renal vascular resistance. IR monitoring helps to diagnose renal failure, determine treatment tactics and predict the outcome of the pathological process in this group of patients. The positive results obtained are due to the stabilizing effect of sodium-glucose cotransporter type 2 inhibitors not only on the functional state of the heart and glucose metabolism, but also directly on renal hemodynamics by reducing swelling of the renal corpuscles and causing positive changes in the renin-angiotensin system. As a result of these pathophysiological processes, vascular resistance decreases and renal hemodynamics stabilizes, which leads to nephroprotective changes, i.e., improvement of renal markers.
References
| [1] | Bongartz L.G., Braam B., Gaillard C.A. et al. Target organ cross talk in cardiorenal syndrome: animal models. Am J Physiol Renal Physiol. 2012; 303 (9): F1253-63. |
| [2] | Cowie M.R., Komajda M., Murray-Thomas T. et al. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). Eur Heart J. 2006; 27 (10): 1216-22. 3-37. |
| [3] | Damman K., Navis G., Voors A.A. et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007; 13 (8): 599-608. 3-41. |
| [4] | Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; Van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]. 2-7. |
| [5] | Forman D.E., Butler J., Wang Y. et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004; 43 (1): 61-7. 3-54. |
| [6] | Gottlieb S.S., Abraham W., Butler J. et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002; 8 (3): 136-41. 3-62. |
| [7] | Hatamizadeh P., Fonarow G.C., Budoff M.J. et al. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013; 9 (2): 99-111. |
| [8] | Kottgen, A.; Russell, S.D.; Loehr, L.; Crainiceanu, C.M.; Rosamond, W.D.; Chang, P.P.; Chambless, L.E.; Coresh, J. Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 2007, 18, 1307–1315. [Google Scholar] [CrossRef]. 2-9. |
| [9] | Krumholz H.M., Chen Y.T., Vaccarino V. et al. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol. 2000; 85 (9): 1110-3. 3-89. |
| [10] | McAlister, F.A.; Ezekowitz, J.; Tarantini, L.; Squire, I.; Komajda, M.; Bayes-Genis, A.; Gotsman, I.; Whalley, G.; Earle, N.; Poppe, K.K.; et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: Impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ. Heart Fail. 2012, 5, 309–314. [Google Scholar] [CrossRef]. 2-8. |
| [11] | Pokhrel, N., Maharjan, N., Dhakal, B. et al., Cardiorenal syndrome: A literature review. // Exp Clin Cardiol, 2008. Vol. 13 (4): P. 165-70. |
| [12] | Rea, M.E.; Dunlap, M.E. Renal hemodynamics in heart failure: Implications for treatment. Curr. Opin. Nephrol. Hypertens. 2008, 17, 87–92. [Google Scholar] [CrossRef]. 2-19. |
| [13] | Struthers, A.D.; MacDonald, T.M. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc. Res. 2004, 61, 663–670. [Google Scholar] [CrossRef] [Green Version]. 2-20. |
| [14] | Torres, V.E. Vasopressin in chronic kidney disease: An elephant in the room? Kidney Int. 2009, 76, 925–928. [Google Scholar] [CrossRef]. 2-21. |
| [15] | Karpov Yu.A., Gendlin G.E., Efficiency of angiotensin receptor blockers at different stages of the cardiovascular continuum — focus on valsartan. Atmosphere. Cardiology news. 2012; 2: 27-31. |
| [16] | Medvedeva E.A., Shilyaeva N.V. et al. Cardiorenal syndrome in chronic heart failure: pathogenesis, diagnosis, prognosis and treatment options. Russian journal of cardiology. 2017; 141(1): 136-141. 3-7. |
| [17] | Reznik E.V. Kidneys as a target organ in chronic heart failure. Lamber. 2011; 188 p. 3-9. |
| [18] | Reznik E.V., Gendlin G.E., Khripun A.I. and others. Functional state of the kidneys, urinary albumin excretion and renal hemodynamics in patients with chronic heart failure. Nephrology and Dialysis. 2010; 12(4): 275-286. 3-12. |
| [19] | Storozhakov GI, Gendlin GE, Reznik EV, The heart is sick - the kidneys suffer: cardiorenal syndrome in chronic heart failure. General Medicine. 2009; 1: 27-36. |
| [20] | Shutov AM, Mashina TV, Marder N.Ya., Chronic heart failure in patients with chronic kidney disease. Nephrology and Dialysis. 2005; 7(2): 140-144. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML