-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(1): 173-176
doi:10.5923/j.ajmms.20251501.32
Received: Dec. 28, 2024; Accepted: Jan. 20, 2025; Published: Jan. 26, 2025

The Interrelation Between Immune Markers and Cytolysis Indicators in Chronic Liver Diseases
Tairova Guzal Babakulovna, Kurbonova Zumrad Chutbayevna
Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Tairova Guzal Babakulovna, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Autoimmune hepatitis (AIH) is a long-lasting liver condition of unclear cause, with symptoms that can vary widely. Most individuals affected by AIH experience elevated liver enzyme levels or show no symptoms at all, leading to a gradual onset of the disease. However, in some cases, it can appear suddenly as acute hepatitis, severe liver failure, chronic hepatitis, or cirrhosis. Jaundice occurs in 115% of patients, and a portion of these individuals may progress to acute or subacute liver failure. The disease is not well documented in medical research, making diagnosis difficult for doctors, as it can be mistaken for other liver disorders and can swiftly lead to severe liver failure. Furthermore, international guidelines and simplified diagnostic criteria are often not very effective at identifying patients within this smaller group.
Keywords: Autoimmune hepatitis, Autoimmune hepatitis liver cirrhosis, Alanine aminotransferase, Aspartate aminotransferase
Cite this paper: Tairova Guzal Babakulovna, Kurbonova Zumrad Chutbayevna, The Interrelation Between Immune Markers and Cytolysis Indicators in Chronic Liver Diseases, American Journal of Medicine and Medical Sciences, Vol. 5 No. 1, 2025, pp. 173-176. doi: 10.5923/j.ajmms.20251501.32.
1. Introduction
- Autoimmune hepatitis develops when the immune system mistakenly attacks liver tissue. This occurs as immune responses, typically directed against external factors like viruses, become activated. However, these responses are more likely to happen in individuals who are genetically predisposed. Type 2 autoimmune hepatitis is a distinct form of the disease, characterized by molecular mimicry, where molecules from viruses or bacteria resemble those found in the body. As a result, the immune system produces antibodies targeting the liver microsomes, specifically the liver/kidney type 1 microsomes. These antibodies then target the liver enzyme cytochrome P450 2D6 (CYP2D6), leading to autoimmune reactions. Consequently, type 2 autoimmune hepatitis is triggered through this molecular mimicry, prompting immune responses against liver enzymes [19]. Brazilian researchers have examined the occurrence of autoimmune hepatitis (AIH) in patients with chronic hepatitis B infection. The study involved liver biopsies from 1,759 patients, with 92 diagnosed with AIH. In addition, among patients with chronic hepatitis C infection, the following findings were observed: 66% tested positive for smooth muscle antibodies (SMA), 41% had liver kidney microsomal (LKM) antibodies, and antinuclear antibodies (ANA) were positive in a number of cases. These findings suggest that hepatitis viruses can coexist with autoimmune hepatitis. This research highlights the possibility that hepatitis viruses might trigger autoimmune responses, contributing to the development of hepatitis. The primary treatments for AIH are corticosteroids and azathioprine, which work to normalize liver enzymes and immunoglobulin G (IgG) levels, ultimately leading to remission—the phase where the disease becomes inactive [4].Additionally, 40% of patients with autoimmune hepatitis (AIH) have a family history of autoimmune diseases, suggesting a genetic predisposition to these conditions. This supports the idea that AIH may have a genetic foundation and could potentially be transmitted within families. While AIH and other autoimmune diseases are linked to genetic inheritance, they do not follow Mendelian inheritance patterns. In Mendelian inheritance, diseases are passed down through genes on specific chromosomes, whereas autoimmune diseases are typically influenced by a combination of multiple genetic factors and environmental influences [20].Therefore, while individuals with a family history of autoimmune diseases may inherit genetic risk factors, environmental influences such as infections, stress, diet, and other factors also play a crucial role. These factors can trigger the immune system to malfunction, contributing to the development of autoimmune diseases. In children, autoimmune hepatitis is mostly linked to genetic predisposition. The relationship between human leukocyte antigens (HLA) and type 1 autoimmune hepatitis was first discovered 30 years ago, marking an important milestone in understanding the genetic underpinnings of the disease [2]. In 1990, Donaldson and colleagues conducted a study to investigate the role of HLA in 96 patients with autoimmune hepatitis (AIH) and 14 patients who had undergone liver transplantation. The study found that AIH patients carried the HLA-DR3 and HLA-DR4 antigens. Those with the HLA-DR3 antigen had a poorer prognosis, with lower treatment efficacy and a greater need for liver transplantation [15].ANA, SMA, anti-LKM1, anti-LC1, and antimitochondrial antibodies were initially identified in tissue samples from the kidney, liver, and stomach. These antibodies were detected using human IgG conjugates as the detection reagents [16].In autoimmune hepatitis (AIH), immune reactions initially triggered by external factors such as viruses and pathogens lead to an immune response in genetically predisposed individuals. Over time, these reactions turn against the individual's own body, specifically targeting autoantigens. The second type of AIH is associated with molecular mimicry, where antibodies are produced against the microsomes of the liver and kidneys, specifically targeting the first type. These antibodies are directed against the liver's CYP2D6 (cytochrome P450 2D6) enzyme [18]. In the treatment of AIH, the cornerstone therapy includes corticosteroids and azathioprine, which help normalize liver enzymes and IgG levels, leading to remission. In 40% of patients with AIH, the presence of autoimmune diseases in the family history supports the genetic inheritance of the disease. [14]. However, inheritance does not follow a Mendelian pattern. In addition to genetic risk factors, environmental factors also play a significant role. In individuals with a genetic predisposition, the influence of external factors triggers the formation of autoimmunity, leading to the development of autoimmune diseases [20]. According to Faisal N. and colleagues, the prognosis for recurrent AIH is favorable; 90% of patients survive after one year, and 86% survive after five years. After transplantation, the recurrence rate of AIH is between 15-40%. Recurrence is more common in AIH-1, where clinical symptoms reappear, ALT and AST levels rise, and autoantibodies and IgG levels increase [8].Moreover, in patients with type 1 AIH who have not received treatment, regulatory T lymphocytes accumulate in the liver. This accumulation forms the basis for the development of recurrence once immunosuppressive treatment is discontinued [12]. Immunosuppressive therapy is widely used in cancer treatment. While these treatment methods have improved the effectiveness of cancer therapy, autoimmune hepatitis is detected in one-quarter of these patients [5]. This type of hepatitis differs histologically from classic AIH [6]. Drug-induced hepatitis is similar to AIH, as both conditions exhibit a similar clinical and histological presentation due to specific immune reactions occurring in liver cells in each disease [3]. In this case, the use of hepatotoxic drugs is a crucial anamnesis factor. Moreover, discontinuing the medication leads to an improvement in the patient's condition, and in some cases, steroid treatment is recommended [7].In patients who have undergone a de novo liver transplant, AIH can develop, presenting similarly to typical AIH. In children, de novo AIH occurs in 4% of cases within 6 to 45 months after liver transplantation, while in adults, this rate is between 1-3%. In the United States, AIH is one of the causes of acute kidney failure. Acute severe AIH is characterized by jaundice, a 1.5-fold increase in HNM, a rise in ANA titers above 1:40, and in 43% of cases, an increase in the smooth muscle antibody (ASMA) titer above 1:40. In 11% of acute AIH cases, ANA and ASMA are negative, and in 15-39% of cases, the levels of immunoglobulins are within normal ranges [1].
2. Materials and Methods
- Research materials and methods. Data were collected from patients treated with autoimmune hepatitis and autoimmune liver cirrhosis in the departments of hematology and hepatobiliary pathology of the 1st clinic of the Tashkent Medical Academy in 2020-2024. Investigations were carried out using Liver-9-Line immunoblot and biochemical methods. Patients in the main group were divided into the following groups. Patients with autoimmune hepatitis type I are included in group 1, patients with autoimmune hepatitis type II are included in group 2. Group 3 includes patients with cirrhosis of the liver with type I autoimmune hepatitis. Group 4 includes patients with type II autoimmune hepatitis and liver cirrhosis, and group 5 includes patients with primary biliary cholangitis.
3. Result and Discussion
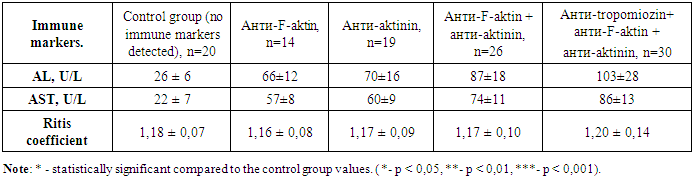
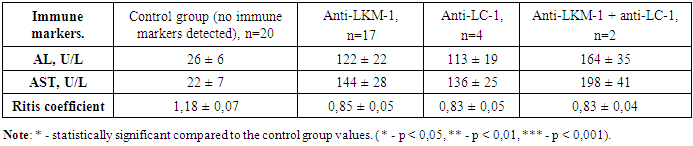
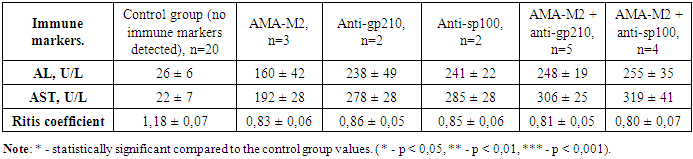
- In chronic autoimmune liver diseases, the study of nine types of antibodies and liver biochemical indicators was conducted using the Liver-9-Line immunoblot analysis. Among 89 patients with type 1 autoimmune hepatitis and type 1 autoimmune cirrhosis in the third group, 63.0% showed positive results for anti-F-actin and anti-actinin markers, 15.7% of the patients had only positive anti-F-actin markers, and 21.3% had only positive anti-actinin markers. The anti-tropomyosin marker showed positive results in 30 patients with type 1 autoimmune hepatitis and autoimmune liver cirrhosis, and in all of these patients, other ASMA group markers, such as anti-F-actin and anti-actinin, were also positive. The results of studying alanine aminotransferase, aspartate aminotransferase, and their ratio are presented in table 1.
|
|
|
4. Conclusions
- In chronic liver diseases, studying the changes in alanine aminotransferase and aspartate aminotransferase levels has shown that the most significant changes occur in primary biliary cholangitis, and these changes were directly related to the number and titer of the detected antibodies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML