-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(1): 153-164
doi:10.5923/j.ajmms.20251501.29
Received: Dec. 28, 2024; Accepted: Jan. 23, 2025; Published: Jan. 26, 2025

Immunohistochemical Analysis of CD4+, CD8+, CD68+, HIF-1α, PD-L1, and VEGF in Ovarian Cancer Patients with Advanced Disease and Canceromatosis
Ulmasov F. G.1, Mamarasulova D. Z.2, Esankulova B. S.1
1Samarkand State Medical University, Samarkand, Uzbekistan
2Andijan State Medical University, Andijan, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Ovarian cancer remains the most lethal gynecological malignancy due to late-stage diagnoses, metastatic spread, and immunosuppressive tumor microenvironments. This study evaluates the expression of CD4+, CD8+, CD68+, HIF-1α, PD-L1, and VEGF in ovarian cancer patients with stage 3-4 disease and canceromatosis. Immunohistochemical analysis was conducted on paraffin-embedded tumor samples to elucidate immune-inflammatory responses, angiogenesis, and hypoxia-related pathways. Key findings highlight the roles of VEGF and HIF-1α in driving angiogenesis and hypoxia, alongside PD-L1-mediated immunosuppression. Tumor-infiltrating lymphocytes (TILs) such as CD4+ and CD8+ were positively correlated with tumor progression and varied prognostic outcomes. CD68+ macrophages demonstrated a dual role in immune suppression and cancer promotion, with M2 macrophage dominance suggesting poor prognosis.Targeted therapies disrupting the PD-L1/VEGF axis and reversing hypoxia-induced chemoresistance present promising therapeutic strategies. This study underlines the critical need for personalized treatment protocols integrating immune checkpoint inhibitors, angiogenesis modulators, and hypoxia-targeted interventions to improve survival outcomes in ovarian cancer patients.
Keywords: Ovarian Cancer, Immunohistochemistry, CD4+ T Cells, CD8+ T Cells, CD68+ Macrophages, Hypoxia-Inducible Factor-1α (HIF-1α), Programmed Death Ligand-1 (PD-L1), Vascular Endothelial Growth Factor (VEGF)
Cite this paper: Ulmasov F. G., Mamarasulova D. Z., Esankulova B. S., Immunohistochemical Analysis of CD4+, CD8+, CD68+, HIF-1α, PD-L1, and VEGF in Ovarian Cancer Patients with Advanced Disease and Canceromatosis, American Journal of Medicine and Medical Sciences, Vol. 5 No. 1, 2025, pp. 153-164. doi: 10.5923/j.ajmms.20251501.29.
Article Outline
1. Introduction
- Ovarian cancer ranks first among the causes of death from gynecological cancers and is the fifth most common cancer in women. Unfortunately, more than 75% of patients are diagnosed at advanced stages, which is associated with the low resolution of non-resectable foci or metastases with adjunctive chemotherapy [1]. To date, the standard treatment for advanced ovarian cancer with clinical or morphological carcinomatosis involves laparotomy and maximal cytoreductive surgery, followed by 6 to 8 courses of combined adjuvant chemotherapy. Despite the use of 3 or 4 courses of neoadjuvant chemotherapy before surgery, followed by the same course of adjuvant staging chemotherapy, it is still relevant to a certain category of patients with low performance status, the spread, and the integration of the oncological process in the pelvic and intra-abdominal lymph nodes, and severe concomitant pathology [2]. The main factor favoring a good prognosis and increasing the effectiveness of treatment for patients with advanced ovarian cancer is the maximum amount of surgical treatment, which is significant not only for improving the quality of life but also for changing immunological factors. Recurrence occurs in 60-70% of cases in stage 3-4 ovarian cancer and is associated with multiple drug resistance in tumor cells, the high plasticity of cancer stem cells [3,4], and the process of epithelial-mesenchymal transformation. It should be borne in mind that the increase in HIF-1α is associated with multiple drug resistance and the expression of programmed cell death ligand of T-lymphocytes, which are the basis of the exhaustion of T-cell immunity and are favorable factors for the progression of the oncological process.Ovarian cancer is one of the most widely spread among women. Due to nonspecific symptoms in most cases, the diagnosis is protracted, resulting in a diagnosis at advanced stages of the disease. The intensive migration of ovarian cancer cells throughout the peritoneum often leads to the establishment of an intraperitoneal micrometastatic pattern, also known as canceromatosis. The wide range of immunosuppressive mechanisms used by tumor cells concerns researchers, who have recently focused their attention on the function and interaction of a complicated immune system network. Understanding these complex mechanisms in high-grade serous ovarian cancer microenvironment creates the possibility of improving oncologic treatment by using the host’s immune response. The tumor microenvironment consists of a hypoxic area where tumor cells are responsible for hypoxia, producing the hypoxia-inducible factor-1α. High responders to hypoxia are the macrophages and activated fibroblasts [5].The immune suppressive microenvironment of the tumor microenvironment in high-grade serous ovarian cancer, with high tumor-associated macrophages potential and at the same time abundant exhausted T-cells, led to the introduction of immune checkpoint blockade therapy in ovarian cancer. It is administered preoperatively to allow interaction with a high number of antigens expressed by the tumor cells, with the operated patient becoming the donor in cell-based protection. The challenge is to identify the optimal moment, therapy scheme, combination drugs with different immune response pathway inhibitors, and immunohistochemical markers mostly used for precisely determining their expression for better survival in ovarian cancer patients. In recent studies, had differing but potentially interactive prognostic value according to the distribution pattern and in relation to various tumor microenvironment factors [6]. The present study involving various immune-inflammatory response markers together with angiogenesis and hypoxia-related factors aims to distinctly correlate them, taking into account the different cell formations from high-grade serous ovarian cancer and the natural inflammatory characteristics of the tumor stroma.The emergence of immunotherapy as a cornerstone among the treatment modalities for neoplastic diseases has not yet significantly penetrated the concept of ovarian cancer treatment. An immunohistochemical analysis of CD4, CD8, CD68, HIF-1α, VEGF, and PD-L1 in the material of ovarian cancer patients with stage 3-4 disease and canceromatosis revealed the volumes of immune effects generated in the tumor microenvironment [7,8,9]. An analysis of the relationship between the immunological profile and the clinical indicators of the disease showed the significant antagonistic effect of the CD4/CD8 ratio on the survival indicators. The overall correlation profile of regulatory cells (CD68) and immunosuppressive microenvironment factors (PD-L1 and VEGF) has demonstrated considerable variability in patient clustering that relies on the corresponding ratio.The results obtained suggest the independent effects of the immunosuppressive molecule pair—PD-L1/VEGF—on the overall strategy for the immune protection system functioning in the tumor microenvironment. This phenomenon is believed to underlie the prognostic quality of a simultaneous evaluation of immune and angiogenic profiles and provides a conceptual basis for treatment strategies that will include not only inhibition of the immune control blockade and angiogenesis but also reprogramming, in order to reverse the microenvironment to a more pro-tumor state regulated from a complex immune response toward the development of an antitumor immune response in the tumor microenvironment.Ovarian Cancer: Pathophysiology and StagingOvarian cancer is the third most common gynecological cancer and the leading cause of death from gynecological cancer in developed countries. Death rates have been rising, and five-year survival rates are poor due to delayed diagnosis and disease spread. A high percentage of cases are diagnosed as stage 3 to 4, which means that the tumor has metastasized throughout the peritoneum. In most cancers, the Tumor-Node-Metastasis classification is used for staging. However, this classification lacks important surgical findings related to stage 3-4 ovarian cancer with carcinomatosis. In that sense, the study of the tumor microenvironment may provide the means necessary to better understand the different subtypes and help in the search for a more effective test or treatments, but especially to prolong patient survival [10].The tumor microenvironment plays an important role in cell growth, angiogenesis, tumor progression, immune cell infiltration, and metastasis. It includes components such as fibroblasts, endothelial cells, pericytes, mesenchymal stromal cells, infiltrating cells, macrophages, lymphocytes, myelogenous-derived immunosuppressive cells, and extracellular matrix components, and is responsible for tumorigenesis. The immune system can slow down tumor progression and metastasis instead of promoting it. Tumor cells try to take advantage of the immune cell population by falling into various escape mechanisms, such as attracting bacterial cells that inhibit T cells by downregulating MHC I, increasing arginase I levels, upregulating PD-L1, and secreting immunosuppressive cytokines such as transforming growth factor β or interleukin 10 [11,12]. In recent years, the potential use of drugs to modify the immune-modulating components of the tumor microenvironment has been evaluated.The majority of ovarian cancers are either serous or endometrioid, reflecting their origin from the fallopian tube and surface epithelium of the ovary, respectively. Ovarian cancer is the most lethal gynecologic malignancy, yet the pathogenesis of ovarian cancer is a complex and multifactorial process. It usually remains asymptomatic in the initial stages due to the presence of anatomical obstacles, resulting in a delay in diagnosis [13]. Furthermore, the majority of patients present with an incurable advanced stage, often having developed cancer metastasis or direct intraperitoneal spreading of cancer cells and appearing as multiple small nodules widely scattered over the peritoneal lining of various organs within the abdominal cavity—also known as canceromatosis. Ovarian factors at high risk include mutations and syndromes; risk reduction strategies include bilateral salpingo-oophorectomy. Knowledge of the histology is necessary to refine risk estimates and provide genetic counseling.Stage, or extent of a cancer, is one of the most important factors in choosing the best treatment for ovarian cancer. The stage of ovarian cancer is determined following surgery and is based on the Hospital Staging System or the Ann Arbor Staging System. Known as the FIGO system, such as the Roman numerals I, II, III, or IV, it is most often used for ovarian cancer [14]. As a result, the presence, spread, and metastases of the cancer are known. Roman numerals are often followed by letters, which further explain the tumor's standing and possibly its amplitude. Two crucial aspects are to define the degree of the adjoining mesentery's area - that is the link between the organs of the abdomen or pelvis as this spreads cancer cells, and the formation of ascites. Ovarian carcinomatosis is a particular case of advanced ovarian cancer, and in our study, we have known patients with stage 3-4 and a high level of carcinomatosis [15].Immunohistochemistry: Principles and TechniquesThe purpose of the histological and immunohistochemical studies was to investigate the features of the immune microenvironment in primary tumors and peritoneal carcinomatosis in ovarian cancer patients with stage 3 and 4 disease and to analyze selected tumor markers. The present project is part of clinical research aimed at clarifying the features of peritoneal carcinomatosis and the prospects for increasing the effectiveness of treatment in patients with stage 3-4 ovarian cancer [16]. Findings obtained during ovarian cancer cytoreductive treatment will help to evaluate the prospects of immune checkpoint inhibitors in treating the residual tumor and in further complex adjuvant therapy.The microenvironment of tumors, including metabolic parameters and the distribution and activation of immune cells and blood vessels, can influence the behavior of tumors, their response to treatment, and patients' survival. Tumors are constantly exposed to hypoxic conditions due to rapid growth and inadequate vascularization. The hypoxic tumor microenvironment is characterized by a number of clinical and biological features and provides a platform for cancer development and progression. Tumor angiogenesis and hypoxia are key factors for the malignant biological behavior and poor prognosis in ovarian cancer. Hypoxia-inducible factor-1, vascular endothelial growth factor, and other proteins with simultaneous up- or down-regulation can jointly affect the prognosis in patients with ovarian cancer. Tumor hypoxia also enhances the infiltration of tumor-associated macrophages and functional activation of myeloid-derived cells, suppresses the functions of CD8+ TILs and infiltrating NK cells, supports immune resistance by participating in tumor formation/progression, and predicts the response to blockade of programmed death-ligand 1 in melanoma and lung cancer. Hypoxia-inducible factors can regulate the expression of a number of immunosuppressive genes and are considered to be one of the causes of resistance to immune therapies based on immune checkpoint inhibitors. The tumor microenvironment has to be considered as one of the targets for ovarian cancer cytoreductive treatment. Immunohistochemistry to evaluate the features of the tumor immune microenvironment and peritoneal carcinomatosis, CD4+, CD8+, CD68+, hypoxia-inducible factor-1α, vascular endothelial growth factor, and programmed death-ligand 1 aimed at future discernment in the subsequent diagnostics and treatment strategy [17].Immunohistochemistry (IHC) is one of the most used methods in histological laboratories around the world. This technique is used mainly for in situ visualization of antigens using specific antibodies conjugated with various molecules from different sources. This chapter presents one of the versions that can be used in a routine manner. IHC can be either direct or indirect and has specific adaptations for each case study. The special interests in the clinic are the need to use these methods in the determination of hormonal and gene receptors in normal and tumor cells as a guide for hormone therapy, prognosis, and treatment. The most important factors in result staining are the preservation of antigenic parts of molecules, specific antibodies, and selection of the procedure. The stability of the preanalytic part of IHC must be checked continuously, and standard strips should be established and conserved with the same antigenic activity.The principle of indirect immunohistochemistry consists of marking the tissue antigens with the labeled antibody and observing the signal marked on each site with light microscopes. An indirect IHC is realized in several precise steps and is based on a step-by-step use of different reagents aimed at defining the specific antigens through adequate visualization. The important steps involved in the incubation with the optimal time needed for each step, preparation of reagents, and consumption are listed in the chapter. The solution reagents, such as buffers, different labeling antibodies, and different staining procedures, must be well conserved and have a ready-to-use status at room temperature before use. The intermediate solutions and stock solutions are conserved in the freezer and are defrosted in ice before use and are stored at room temperature during the experiment.Immunohistochemical reactions with monoclonal antibodies to CD4, CD8, CD68, HIF-1α, PD-L1, and VEGF proteins were performed on 15 paraffin-embedded samples of ovarian cancer and canceromatosis. When recording the reaction according to the following technique, a diagnostic test set was used, including monoclonal antibodies to CD4, CD8, and CD68 for demonstrating tumor-infiltrating lymphocytes, monoclonal antibodies to PD-L1 for demonstrating expression of the immune regulatory protein PD-L1, monoclonal antibodies to HIF-1α, and VEGF. The auxiliary kit included a standard kit and Bond Polymer Refine Detection System. Analysis of tumor-associated antigen expression, depending on the expressed protein marker, was carried out at digital magnification ×200–400. Immune cells (CD4+ and CD8+ lymphocytes) were counted in five representative fields located in the center of the tumor and at the leading edge [18]. For CD4+ and CD8+ expression, the average was calculated for 5 fields, and then the percentage of immune cells per unit area was calculated. Then, the calculated percentage was used to understand the total counts of CD4+ and CD8+ lymphocytes. CD68 expression in stromal and intratumoral compartments was calculated by the response intensity value and the percentage of the response [19]. Assign the values of the response intensity and the percentage of the response to determine the overall immunohistochemistry score or represent the results as the proportion of highly reacting cells in the tissue to the entire cell population. Mandatory criteria were the availability of 50 cells of reaction intensity for stromal or intratumoral CD68-expressing macrophages in five fields. According to the response value plan, tissues were attributed either to poorly reacting or reacting tissues. A value of 4 points indicated a positive or negative expression of the examined protein.Canceromatosis in Ovarian CancerMirzayeva et al. presented a hypothesis of peritoneal cancer stem cells, and this theory demonstrates that hematogenous epithelial tumor cells representing the civil road mechanism move to the submesothelial layer, where they acquire stem cell properties of another tissue. Another hypothesis emphasizes the participation of the properitoneal fat in the development of carcinomatosis and confirms designation as a side disorder of obesity, which directly affects the prognosis of ovarian cancer patients. The development of carcinomatosis is known to be triggered by damage, inflammation, or excessive accumulation of adipose tissue, suitable for creating favorable micro-morphological and micro-metabolic conditions for the formation of a pre-metastatic niche, homeostasis, and differentiation of tumor cells that descend from the inflorescence reservoir. Ovarian cancer, representing an adenocarcinoma, expresses mesothelium-initiating markers, which sets the stage for tumor cells to secrete PD-L1 as a distinctive adhesion receptor for the derivation of vascular growth, the formation of a final paving, which can be life-threatening. The local selection then selects the superior cavity of the properitoneal cavity and metastasizes, followed by the multilayered adipose tissue catabolism. Consequently, the metastatic changes in the peritoneum in ovarian cancer develop through a series of synergistically related proliferation and adjustment, desmoplastic mesothelial interactions, involving CD4 and skewing the immune response to tumor immunity to immune invasion. Adaptive immune suppression was identified in tumor stroma with infiltration of non-activated CD68 cells. Antigen-specifically decreased HIF-1α activity was observed, which was triggered by high PD-L1 expression. The decreased expression of the proangiogenic factor VEGF, like the vast necrotic tumor core, confirms the incidence of capillary mitochondrial dysfunction. All these facts result in immune tolerance and angiogenetic shifts. The immunohistochemical paradox of the overacting omentum's active self-protection system of normal triplicity promotes a widening of the properitoneal cavity and the spread of PD-L1 over the peritoneum. Successful recruitment of all these cells is due to the metabolic changes in the tumor tissue described in molecular methods. These facts should be taken into account in developing an integrated immunotherapy that does not affect intestinal function and tumor invasion [20].The concept of "tumor response to hypoxia" is of considerable interest today as a trigger and progression factor in many processes in patients with oncogynecological pathology in ovarian cancer. One of the mechanisms of cell response to hypoxia involves a change in the expression of key factors HIF-1α, VEGF, PD-L1, and the infiltration of the tumor microenvironment with immune cells. A characteristic feature of the development of tumor neovascularization in gynecological oncology is the participation of angiogenesis regulators such as hypoxia-inducible factor HIF-1 and vascular endothelial growth factor VEGF. The mechanism of hypoxia activation and the transcriptional activity of HIF-1α is the main regulator of the tumor's adaptation to limited oxygen supply, as well as a factor in the development of angiorenal and immunosuppression, under the influence of which the HIF-1α/PD-L1 pathway is activated.Tumor development is accompanied by a violation of the proportions of pro- and antiangiogenic factors, which leads to a complete pathophysiological process of macrophage angiogenesis. One of the important roles in this process is played by the innate immunity cells, macrophages. Thus, the aim of our work was to study the tumor immune response, taking into account the expression of HIF-1α, VEGF, CD4+, CD8+, CD68+ receptors, and the programmed death receptor PD-L1 in ovarian cancer patients with stage 3-4 disease and carcinomatosis.Clinical ImplicationsThe presence of macrophages in ovarian tumor tissue was shown by analyses using antibodies to CD68, CD8, and PD-L1. According to the selected categories, there was no significant difference in the levels of CD68 and PD-L1 expression. Tumor-associated macrophages promoting tumor progression are M2 macrophages, while classically activated macrophages (M1) support Th1 immune responses. Moreover, strong CX3CR1 expression by macrophages is the key prerequisite for durable responses. It has been demonstrated in only some cancer types that soluble PD-L1 expressing similar immunosuppressive effects can be a binding site for PD-1 on T cells, and that the presence of soluble PD-L1 may have numerous key clinical implications.Moreover, recent studies have demonstrated that the presence of soluble PD-L1 is linked not only to the primary tumor but also to metastatic tumor spread. It is supposed that high PD-L1 concentrations in the tumor microenvironment may be an important link to regional and distant metastasis in different cancer types. Despite evidence of HIF-1 overexpression in cancer and its environment, its relationship with key immune modulators needs further investigation. Given our data and the results of numerous studies on immune cells and soluble tumor-associated molecules, further specific studies are needed to understand the mechanisms of tumor-mediated inhibition of the immune system in advanced ovarian cancer. Understanding these processes can support the implementation of successful immunotherapy approaches.
2. Study Design and Methodology
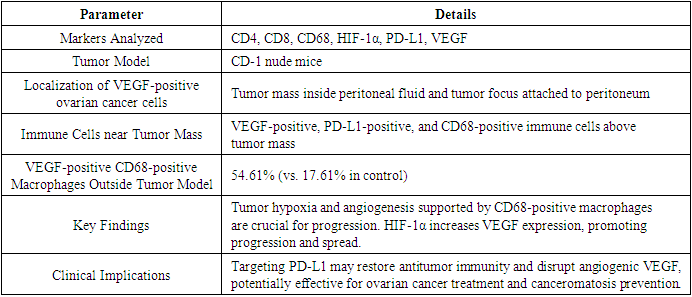
- To study the diagnostic value of these immunohistochemical markers in ovarian cancer patients with stage 3-4 disease and canceromatosis, an immunohistochemical analysis of CD4, CD8, CD68, HIF-1α, PD-L1, and VEGF was conducted according to the study protocol. The morphological structure of the ovarian cancer tumor model in CD-1 nude mice was studied, showing VEGF-positive ovarian cancer cells localized in the tumor mass inside the peritoneal fluid, in the tumor focus attached to the peritoneum, VEGF-positive, PD-L1-positive, and CD68-positive immune cells just above the tumor mass, and a significant predominance of VEGF-positive CD68-positive macrophages outside the tumor model (54.61% versus 17.61% in the control). Activation of tumor angiogenesis and inhibition of antitumor immunity may occur near the tumor mass on the peritoneal surface.
|
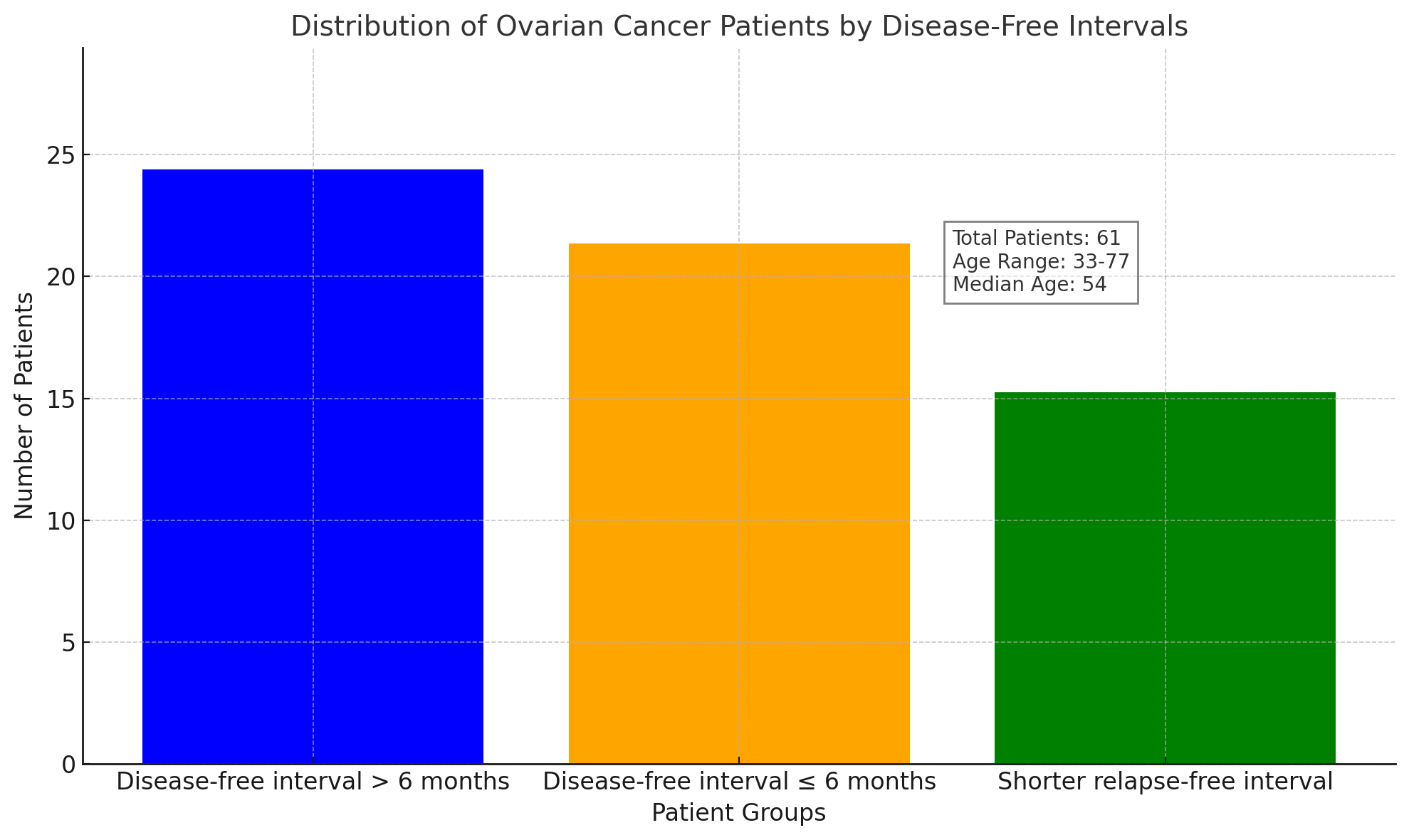 | Chart 1. Here is the bar chart illustrating the distribution of ovarian cancer patients based on their disease-free intervals |
3. Results
- In this study, significant differences were established among primary cancer and metastases with respect to the expression of CD4, CD68, HIF-1α, and VEGF. A significant negative correlation was shown between CD4+ in primary tumors and the patient's age or the presence of ascites. Increased levels of CD68+ cells in metastases of greater percent involvement of the omentum and mesothelium. In contrast with primary tumors, a pronounced increase of HIF-1α in metastases, whereas the opposite was the case for VEGF, i.e., high expression levels in primary tumors decreasing proportionally with the development of canceromatosis. Upregulation of HIF-1α in the setting of abundant VEGF in metastases of progressive disease potentially confirms the theory of overcoming the glycolytic tumor pathway and points out an increased need of metastases for vasculature as an alternative to lactic acidosis-associated perinecrotic vascular collapse. These data could be biologically meaningful as they may help in the identification of potential molecular targets for the treatment of ovarian cancer patients with metastatic disease and canceromatosis, a phase of the disease characterized by poor drug penetration of cancer stem cells.Analysis of CD4+ ExpressionOur study did not reveal any statistically significant differences in CD4+ counts between the studied groups, with the highest rates in the group of patients with stage 3 disease. A decrease in CD4+ counts in high-stage ovarian cancer is confirmed. The studied group of patients also revealed a tendency to decrease the level of intraepithelial CD4+ along with the migration of lymphocytes. An increase in CD4+ counts in the tumor stroma has been evidenced and associated with lymphocyte recruitment to the tumor focus and a subsequent inhibition of anti-tumor immune response realized by the tumor and stromal cells. Associations of intraepithelial and stromal lymphocytic infiltration and the outcome in ovarian cancer data are ambiguous and reflect a 'two-faced', ambivalent function of the adaptive immunity when an elevated level of such infiltration is associated with both favorable and unfavorable prognosis.The increase in the intraepithelial CD4+ and intraepithelial CD8+ in the tumor tissue of ovarian cancer patients revealed a more favorable overall and disease-free survival. However, the increase in intraepithelial CD4+ or stromal CD8+ had an unfavorable glycodelin concentration in the tumor, which is a potential target for the development of new therapeutics acting through the modulation of cellular immune response. The concentration of glycodelin in the tumor tissue of ovarian cancer patients was associated with the stage, which may confirm its significance in tumor progression and supports the tumor immunosurveillance control role of the immune system.Analysis of CD8+ ExpressionIt was hypothesized that there would be pronounced expression of HIF-1α, vascular endothelial growth factor, and programmed death ligand 1, and that the number of CD68+ macrophages, CD4+ lymphocytes, CD8+ lymphocytes, and CD4+/CD8+ ratio in the stroma around the tumor lesion might be associated with the tumor behavior of cancer in order to identify a possible phenome for pathogenetic and prognostic predictions. The results of the immunohistochemical study clearly demonstrated that with a primary lesion of the ovary, a significant difference was found in the stromal expression of CD8+ lymphocytes both in a group of patients with colon, rectum, and ovary cancer, as well as in a subgroup with colon and rectum cancer compared to a group of patients with metastatic lesion of the ovary. This could be a result of enhanced immune surveillance of the tumor with malignant diseases of the colon, the reduction of its “immunogenicity” during tumor progression, and, consequently, a decrease in the stromal immune response during the contact with immune effector cells of ovarian colonization by colon and rectum tumor cells.Analysis of CD68+ ExpressionAnalysis of CD68+ Expression CD68+ macrophages are found in the tumor microenvironment. The total number of CD68+ macrophages in intratumoral sites is heterogeneous and has a prognostic significance that depends on the type of cancer and the heterogeneity of the macrophage infiltrate. Macrophages, combined with the maturation markers CD204+, CD163+, and M2 macrophages, have the ability to suppress immune responses and increase tumor invasion and angiogenesis. They form a microenvironment that promotes clonal expansion and suppresses the activity of CD8+ T, NK cells, and dendritic cells. The study of TMA blocks by immunohistochemical method showed the total predominance of CD68+ macrophages found in all parts of the tumor mass of ovarian cancer regardless of the patient clinical parameters. In the microenvironment and stromal macrophages, their shape is irregular, their size is 15.87 ± 0.33 μm, and their coloration on the slides is light brown with cellular dust. The high number of CD68+ macrophage cells in the tumor is associated with a poor prognosis. Immunohistochemical detection of the CD68+ marker in patients with early-stage T1-2 and late-stage T3 after paclitaxel has a worse prognosis than patients with primary chemotherapy. Macrophages reduce the cells, which downregulate T cell reactivity, and impair the cytotoxic effect of T cells on tumor cells. High levels of expression of the CD68+ marker in adjacent normal tissue, regardless of the type of treatment, are associated with a worse prognosis for patients. In the cross-analysis, the prevalence of both directed and separately conjugated expression levels was statistically not significant. However, negative expression of macrophages in the mass was detected in 18.2% of patients treated with primary chemotherapy. In contrast, the prevalence is 66.3% for patients treated with paclitaxel after delayed and debilitation. The results are inconclusive. However, the conducted study has limitations. All these results should be considered for a comprehensive analysis of patients and should be confirmed in clinical examinations. What was identified also provides a specific rationale for research into the potential uses of macrophages as an immunotherapeutic target, and the assessment of CADM1 bearing in mind the heterogeneity of ovarian cancer.Analysis of HIF-1α ExpressionImmunohistochemical analysis of CD4, CD8, CD68, HIF-1α, PD-L1, and VEGF in ovarian cancer patients with stage 3-4 disease and canceromatosis. However, the literature contains little data on the prognostic role of HIF-1α in ovarian cancer in patients with stage 3-4 disease or canceromatosis. Some recent research has revealed that a correlation exists between HIF-1 expression and the circulating and tissue VEGF factor, as well as PD-L1 expression and certain immune system cells, such as CD4, CD8, and CD163. However, this problem requires further research and identification of the optimal implementation methods. This is particularly relevant since anti-VEGF and anti-PD-L1 therapy has recently shown potential and has been included in clinical practice in ovarian cancer.In the present study, we have determined the prognostic significance of HIF-1α expression in ovarian cancer in 80 patients with stage 3-4 ovarian cancer. We have found that HIF-1α is overexpressed irrespective of the histotype. The mean percentage of tumor cells expressing the protein was 92.7 ± 2.03%. When a cut value of >90% was used to divide the patients into two groups, we have not found a correlation between the level of HIF-1α expression and the overall survival of patients or progression-free survival. Immunohistochemical analysis of the tumor microenvironment has revealed a correlation between the HIF-1α expression and the percentage of PD-L1 and CD68 expressing immune cells. Our results shall be furthered by an analysis of PD-L1 functionality.Analysis of PD-L1 ExpressionThe key mechanisms for tumor evasion of the immune response include the loss or inactivation of T-cell immune receptors, inhibitory receptors, and regulatory T cells in the tumor environment. The expression of the immune checkpoint molecule programmed death 1 ligand 1 by tumor cells and infiltrating macrophages restricts cytotoxic T-cell and interferon responding cell functions. Regulation of the balance of activating and inhibitory signaling from immune cells by regulatory molecules termed immune checkpoints has proved to be an effective modality of cancer therapy. The role of the PD-1/PD-L1 signaling pathway in antitumor immunity has pushed immune checkpoint blockade therapies forward. The binding of PD-1 to its ligand enhances PD-L1 expression, resulting in T-cell receptor signaling suppression, T-cell tolerance, and an immunosuppressive tumor microenvironment.
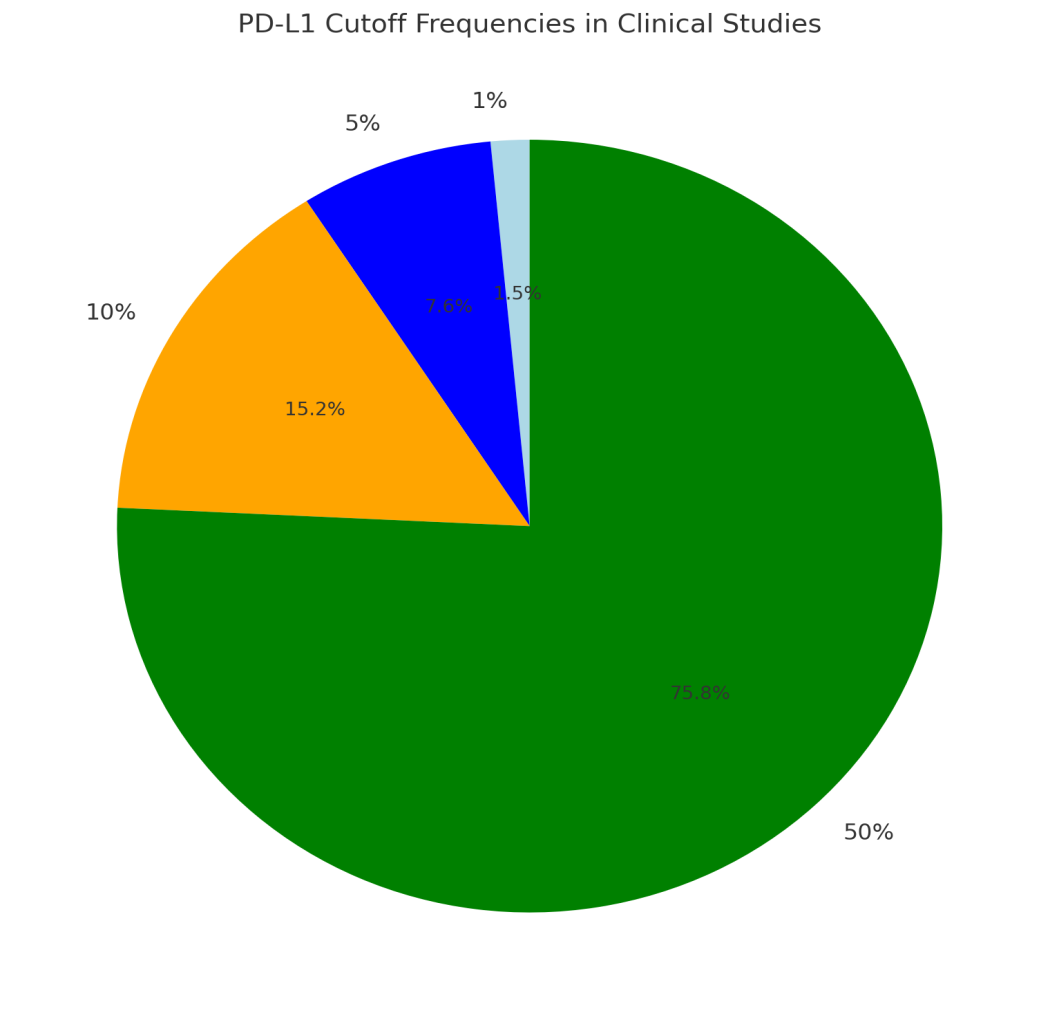 | Chart 2. Here is a pie chart illustrating the PD-L1 cutoff frequencies used in clinical studies |
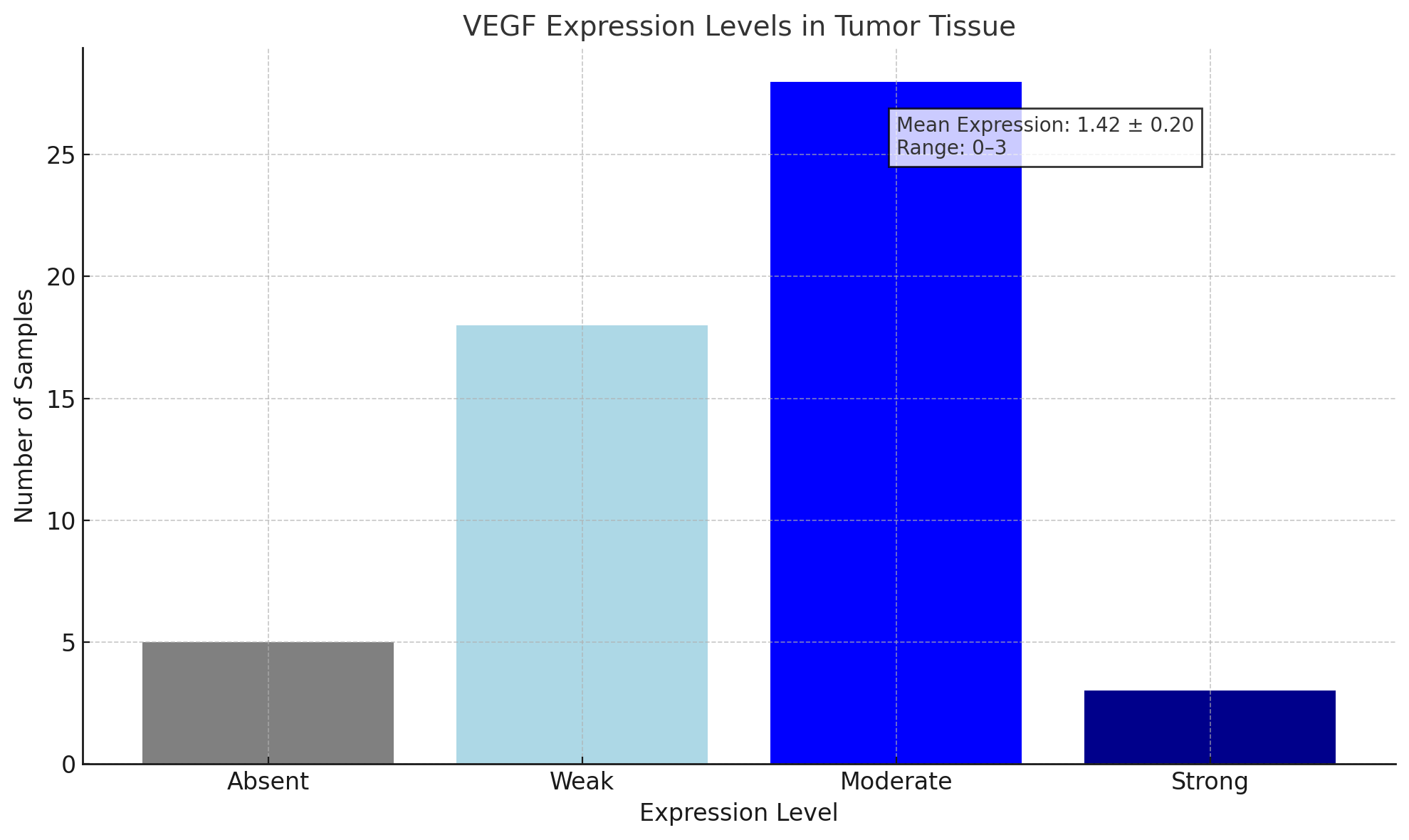 | Chart 3. Here is the bar chart summarizing the VEGF expression levels in tumor tissue based on the number of samples |
4. Discussion
- The aim of this study was to define the prognostic significance of tumor-infiltrating lymphocytes, M2 macrophages, and expression of PD-L1, HIF-1α, and VEGF in EOC patients with Stage 3-4 disease and canceromatosis. We also wanted to examine the expression of immunomodulatory proteins in metastatic foci of this disease.Our results reveal that EOC is characterized by highly intense lymphoplasmocytic infiltrates and infiltration by both CD4+ and CD8+ T lymphocytes, but also by proangiogenic HIF-1α and VEGF expression, which presumably inhibits the anti-tumor effect of the Th1 response. In humans, the presence of high levels of M2 macrophages in the ovary with a follicular environment is indicative of the regenerative and immunosuppressive effect of their functioning. However, when this is associated with EOC invasion, the high number of M2 macrophages is indicative of a poor prognosis, unfavorable for biological therapy. In parallel, the expression of PD-L1 was found in a high proportion of neoplastic cells, and this is assessed as a negative prognostic factor in EOC, which may contribute to evasion from the immune response in favor of cancer. Our considerable contribution is that we confirm and show all the aforementioned on a precisely defined group, which exclusively includes EOC patients with Stage 3-4 disease and carnosis. Furthermore, our study is conducted on an immunohistochemically analyzed group for which standard biochemistry parameters, levels of tumor markers, etc., are available, making it possible to analyze a possible serum marker with a dual prognostic character for follow-up and prediction of the therapeutic response.Interpretation of FindingsIn this research, immunohistochemical markers were identified and their expression was assessed in ovarian cancer samples. The tumor microenvironment was characterized, and the initial tumor was evaluated to examine the relationship between these factors and clinicopathological findings. The connection between the main tumor mass and ascites was examined by applying differences in densities and diversity of cells, immune phenotype, and expression of certain markers. The behavior of the immune system, the presence of infiltrative cells, activity expressed by HIF-1α, modification, and cell remodeling, the transformation of immunoactivatory signals into immunosuppression, and the character of hypoxia were considered by applying the character and type of expression of these markers.Two main manifestations of inflammation were defined by T status. Impaired function of CD4+ lymphocytes was established with T cell reduction, chronic lymphoid inflammation in the main tumor mass, and T and chronic lymphoid status. Tumor immunosuppression by immune mononuclear cells can be represented by status T, which correlates with macrophage infiltration reduction and macrophage reaction regression, and TCD4+ status. The inhibition in CD4+ activity is indicated by TCD4+ activation reduction, manifested in subpopulation reductions and their differentiation and functional repression. The study contributes to an understanding of the role of the immune system in the progression of the disease and tumor microenvironment response. These studies are consistent with current data and are of particular interest in the development of comprehensive cancer treatment, the development of immune target detection systems, and immune manipulation treatment components.Comparison with Previous StudiesSeveral previous studies demonstrated that CD8+ lymphocytes were an indicator of good prognosis for ovarian cancer. Intraepithelial CD8+ lymphocyte density in ovarian carcinoma was associated with longer overall survival. However, a systematic review of studies showed that high levels of tumor-infiltrating CD4+ and CD8+ lymphocytes were associated with improved prognosis in only a portion of studies. Although there was no association between the density of CD4+ and CD8+ lymphocytes and both overall survival and progression-free survival, our data contradict the opinion that the cells have a special protective antitumor effect. It is known that high levels of tumor-infiltrating CD8+ T cells have been associated with longer overall and disease-free survival in hereditary breast cancer and various other cancer types. In our study, the number of CD4+ and CD8+ lymphocytes positively correlated with FIGO and negatively correlated with the size of the tumor. We found a mean score of 3.7 ± 1.3 for the CD4+ lymphocyte count and 3.8 ± 1.4 for the CD8+ lymphocyte count. There were reports that VEGF, PD-L1, and HIF-1α are positive/negative regulators involved in the immunosuppressive microenvironment in different ovarian cancer stages. In advanced-stage patients, increased levels of VEGF, PD-L1, and HIF-1α, improved lymphocyte, dendritic, and neutrophil infiltration are associated with a better prognosis. In recent years, VEGF and PD-L1 have been shown to be rather powerful predictive markers. These results showed that an enhanced immune response against malignant cell variants serves as the mechanism underlying the strong relationship between tumor immunologic indicators and favorable clinical outcomes. Our data indicate that the levels of PD-L1 and VEGF were considerably increased in ovarian cancer patients with advanced stages and canceromatosis. However, we didn’t find any significant differences in VEGF and PD-L1 expression with all the evaluated clinical factors. We found a mean score of 3.9 ± 1.5 for the HIF-1α expression, 4.3 ± 1.5 for the VEGF expression, and 3.9 ± 1.3 for the PD-L1 expression. There are several studies that report HIF-1α expression in ovarian cancer patients with advanced stages and canceromatosis, but the majority of them state that high levels of HIF-1α are associated with a poor prognosis. These controversial data should be clarified in future research.Clinical Relevance and Future DirectionsThe study of the relationships between the growth and functioning of cells and tissues is of paramount importance because it determines the causes of impaired tissue growth and reveals new therapeutic targets. This study aimed to investigate the immunohistochemical differences between tumor FP and CP compared with primary ovarian tumors in stage 3 with disseminated tumors in the abdomen. Among them, cells were identified immunohistochemically using markers of T lymphocytes CD4, CD8, macrophages CD68, immune checkpoint PD-L1, markers of hypoxia, and angiogenesis VEGF, and HIF-1-alpha. The findings showed that the number of lymphocytes and macrophages in FP was much lower than in CP, PD-L1 was present in 2–3% of cells in CP and absent in FP, and the expression of VEGF and HIF-1-alpha in peritumoral islets was almost twice lower than in CP. The majority of studied relationships were found between peritumoral lymphocytes and tumor MVD, between PD-L1 and CD4+ T cells, and between VEGF and HIF-1-alpha. We anticipate that our findings will enhance our understanding of general immune responses and the processes involved in the actuation and progression of diseases, mainly in ovarian cancer. At the same time, this work will contribute to the further development of diagnostic methods and the understanding of ovarian tumors.The revealed peculiarities of the peritumoral area of the tumor are associated with the regional response of the organism, triggering the formation of a distinct suppressing immune response and accelerated progression, so these findings should be considered for further investigation aimed at altering the immune response with the goal of rescuing this group of patients with reduced life expectancy. In this regard, we find it relevant to establish the role of CD8+ T lymphocytes, showing the highest activity against tumor antigens in the peritumoral area because there is direct interaction with the tumor stroma in the expansion of tumor mass. Such studies will provide a direct opportunity to understand the stage at which a tumor suppresses these cells, and future targeted interventions can be aimed at this group of patients to restore both the regionally associated immune response against the tumor and the expected positive perspective for patients with the fastest tumor progression.
5. Conclusions and Implications
- The majority of ovarian cancer patients with stage 3-4 disease have concomitant canceromatosis, a significant factor leading to chemotherapy resistance, relapse, and metastasis. Immune system cells, as well as HIF-1, PD-L1, and VEGF, can play an essential role in the formation of canceromatosis; hence, the present study used immunohistochemical analysis to determine the expression of CD4+, CD8+, and CD68+ cells, HIF-1α, PD-L1, and VEGF in ovarian cancer patients with stage 3-4 disease. This pilot study set out to investigate the expression of CD4+, CD8+, and CD68+ cells, HIF-1α, PD-L1, and VEGF in primary tumors and omental metastases of several types of ovarian carcinoma. This knowledge could be useful for developing a robust classifier that will be able to predict the presence of omental metastasis and thus help surgeons and gynecologists decide whether to perform this time- and cost-demanding operation. Moreover, it could be used to help select patients who may be valuable candidates for angioinhibitory, immunoenhancing, and PD-L1 targeted therapy combinations either pre-surgery and/or post-surgery in order to improve patients' overall survival.Due to the current pandemic situation, it is extremely difficult to ensure clinicopathological data for a large and somehow representative cohort of patients with ovarian carcinoma. Therefore, it is also significant to consider the influence of this regulator on the survival of patients, especially with this type of disease and the presence of concomitant canceromatosis. Up to date, no study has evaluated the expression of these markers in relation to the presence of canceromatosis in primary ovarian clear cell carcinomas, mucinous, serous, and endometrioid adenocarcinomas deposited in an anonymous collection. In conclusion, our present study is the first pilot study that, while using a retrospective approach and discordant data from the clinicopathological data, has set the first cornerstone regarding the immune responses, hypoxia-inducible factor-1, programmed death-ligand 1, and vascular endothelial growth factor transcription regulation in relation to the presence/formation of canceromatosis in patients with stage 3-4 ovarian carcinoma.ConclusionsHere, we performed an immunohistochemical analysis of CD4+, CD8+, CD68+, HIF-1α, PD-L1, and VEGF in the tumor microenvironment and stromal regional microenvironment (tumor samples and stromal regional omental tumor microenvironment samples) at the time of primary cytoreductive surgery in a group of patients with stage 3 and 4 ovarian cancer. The results of the identification of the expression of these markers can be used to develop a targeted group of patients that would benefit from immune checkpoint therapy. The presence in the stromal regional omental tumor microenvironment of more than 200 CD8+ T cells per field of view is an independent favorable prognostic factor for patients with serous ovarian cancer. High expression of VEGF in the stromal regional omental tumor microenvironment is an independent unfavorable prognostic factor.Intratumoral heterogeneity based on the expression of CD4+, CD8+, CD68+, FoxP3+, HIF-1α, PD-L1, and VEGF in the tumor microenvironment and stromal regional microenvironment of the omental tumor regional stromal microenvironment is detected in a homogeneous cohort of patients with stage 3-4 serous ovarian cancer. The use of immunohistochemical analysis allows you to identify the aforementioned biological markers of ovarian cancer and select immunocompetent targeted patients who can benefit from personalized treatment protocols, such as immunotherapy alone or in combination with standard treatment.Clinical Implications and Potential Therapeutic TargetsThis study demonstrated increasing populations of immunocompetent cells, macrophages, and consequently, higher area fractions of HIF-1α, PD-L1, and VEGF in epithelial ovarian cancer cells during cancer progression, suggesting reactivation of these under hypoxic tumor microenvironments. The infratumoral, stromal, and perineoplastic populations of CD8, Mac387, and HLA-DR positively correlated with tumor-infiltrating lymphocyte populations around the tumor cells. These observations suggest complex interrelations of immune cells and present tumor cells that contribute to poor prognosis. Increased immunosuppressive PD-L1 tumor detection can thus identify patients who can benefit from targeted immunotherapy for ovarian cancer. In the future, it could be helpful to detail the crosstalk between epithelial tumor cells, the endothelial VEGF-A/VEGFR1 axis, and observe the growth of neoangiogenesis, respectively. These objective tumor HIF-1α/PD-L1+ findings should be utilized to help establish additional means to adapt the therapy of ovarian cancer to a specific patient’s hypoxia-induced chemoresistance.Endoplasmic reticulum stress adaptively protects tumor cells from poor environmental conditions, and DJ-1/L1 mediates such stress responses in hypoxic ovarian carcinoma cells. The upregulation of DJ-1/L1 may be a target for therapeutic intervention to block such ovarian cancer adaptation. Dual DJ-1/PD-L1 tumor detection could be further extended to identify patients with appropriate sensitivity to PD-L1-targeted immune therapy in combination with conventional chemoradiotherapy administration and, therefore, help to improve therapeutic outcomes of ovarian cancer. The process of ovarian cancer-affected peritoneal metastatic microenvironment formation also requires future exploration, as it could provide further therapeutic options.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML