-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(1): 1-8
doi:10.5923/j.ajmms.20251501.01
Received: Dec. 4, 2024; Accepted: Dec. 23, 2024; Published: Jan. 11, 2025

Evaluation of Safety and Tolerability of a Single Application of 5% Povidone-Iodine Solution for the Treatment of Adenoviral Conjunctivitis
Kuvondikov Elyor1, Fayziyeva Dilorom2
1Samarkand Regional Military Hospital, Otorhinolaryngology Department, Samarkand, Uzbekistan
2Department of Combat Surgical Pathology, Military Medical Academy, Tashkent, Uzbekistan
Correspondence to: Kuvondikov Elyor, Samarkand Regional Military Hospital, Otorhinolaryngology Department, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective: To evaluate the safety and tolerability of a single application of 5% povidone-iodine (PVP-I) solution in a double-blind, randomized clinical trial for the treatment of adenoviral conjunctivitis (Ad-Cj). Methods: Of the 212 patients screened, 56 participants presenting with symptoms of eye redness lasting ≤4 days and a positive adenovirus rapid test were included in the study. Participants were randomly assigned to receive either a single application of 5% PVP-I or preservative-free artificial tears (AT). Safety was assessed through fluorescein corneal staining at baseline, immediately after treatment, and on Day 1, along with visual acuity (VA) testing at baseline and Day 1. Tolerability was evaluated based on participants' self-reported discomfort at baseline, immediately after application, and on Day 1. Results: In the 5% PVP-I group, an increase in corneal staining was observed immediately after application, but levels returned to baseline by Day 1. Visual acuity remained unchanged between baseline and Day 1 in both groups (p = 0.87). Participants in the 5% PVP-I group reported no significant changes in discomfort immediately after application (p = 0.78) or on Day 1 (p = 0.10) compared to baseline. In contrast, the AT group experienced temporary relief from discomfort immediately after application, which returned to baseline levels by Day 1. Conclusion: The findings indicate that a single ophthalmic application of 5% PVP-I is safe and well-tolerated for patients with adenoviral conjunctivitis.
Keywords: Povidone-iodine, Adenoviral conjunctivitis, Corneal fluorescein staining, Artificial tears, Visual acuity, Safety, Tolerability, Ophthalmic antiseptic, Ocular discomfort, Adverse events, Preservative-free artificial tears, Ophthalmology, Dry eye symptoms, Antiviral therapy
Cite this paper: Kuvondikov Elyor, Fayziyeva Dilorom, Evaluation of Safety and Tolerability of a Single Application of 5% Povidone-Iodine Solution for the Treatment of Adenoviral Conjunctivitis, American Journal of Medicine and Medical Sciences, Vol. 15 No. 1, 2025, pp. 1-8. doi: 10.5923/j.ajmms.20251501.01.
Article Outline
1. Introduction
- Adenoviral conjunctivitis (Ad-Cj) is a highly contagious condition that can spread rapidly in clinics, homes, schools, and workplaces, leading to significant morbidity and economic burden. This condition poses particular challenges in healthcare and educational settings due to its ease of transmission and potential to disrupt daily activities. Current guidelines, such as those from the American Academy of Ophthalmology and the American Optometric Association, recommend supportive therapy, including artificial tears (AT), topical antihistamines, and cold compresses [1,2]. However, there is currently no approved treatment for Ad-Cj, underscoring an unmet medical need for effective and targeted therapies. Alternative approaches are being investigated, including the single application of a 5% povidone-iodine (PVP-I) ophthalmic solution, which has shown promise in preliminary studies [3–6]. A 2013 survey of eye care professionals revealed that approximately one-third of respondents reported off-label use of 5% PVP-I for treating Ad-Cj [7]. Despite this, there has been no systematic evaluation of the safety and tolerability of this approach until now. The ophthalmic formulation of 5% povidone-iodine (PVP-I) (Betadine® 5) is officially approved for “preparation of the periocular area (including eyelids, eyebrows, and cheeks) and irrigation of the ocular surface (cornea, conjunctiva, and conjunctival fornices)” [8]. This product is widely utilized in ophthalmic surgery to prevent endophthalmitis [9,10]. PVP-I exhibits potent antiseptic properties, effectively targeting a broad spectrum of pathogens, including bacteria (both intracellular and extracellular chlamydia), fungi, protozoa, and viruses such as adenovirus, herpesvirus, and enterovirus. Additionally, it has demonstrated minimal toxicity to the cornea and other ocular structures, making it a promising candidate for broader ophthalmic use [11].
1.1. Global Context and Clinical Significance
- Adenoviral conjunctivitis represents a substantial global health challenge, particularly in developing countries where access to specialized ophthalmic care is limited. The highly transmissible nature of the disease often leads to outbreaks, placing a strain on healthcare systems. Identifying a safe, effective, and accessible treatment option could significantly reduce the burden of this condition worldwide.
1.2. Rationale for the Study
- Given the current reliance on supportive therapy and the off-label use of PVP-I without rigorous clinical evaluation, this study aims to systematically assess the safety and tolerability of a single 5% PVP-I application. This approach has the potential to bridge the gap between supportive care and targeted antiviral treatment, addressing an important unmet need in ophthalmology.The efficacy and safety of PVP-I at various concentrations [12–14], as well as its combination with steroids, are currently being investigated in randomized clinical trials for the treatment of adenoviral conjunctivitis (Ad-Cj) [15–18]. However, the safety and tolerability of the ophthalmic formulation of 5% PVP-I in patients with Ad-Cj have not yet been comprehensively studied. Therefore, double-blind, randomized, placebo-controlled clinical trials are necessary to systematically evaluate the safety and tolerability of this treatment approach.
2. Methods of Study
2.1. Stydy Disign
- This study was designed as a double-blind, randomized trial to collect preliminary data for a subsequent definitive clinical trial assessing the safety and efficacy of a 5% povidone-iodine (PVP-I) solution for treating adenoviral conjunctivitis (Ad-Cj). Participants with acute Ad-Cj symptoms were randomly assigned in a 1:1 ratio to receive either a single application of 5% PVP-I or preservative-free artificial tears (AT) during their first visit. Randomization was achieved through a computer-generated sequence to ensure allocation concealment. Both interventions were provided in identical packaging to maintain blinding for participants and investigators.
2.1.1. Study Population
- The inclusion criteria required participants to have symptoms of conjunctivitis lasting ≤4 days and a positive result on the adenoviral rapid test. Key exclusion criteria included hypersensitivity to iodine, a history of ocular surgery within the past 3 months, concurrent ocular infections, or the use of topical ocular medications within 48 hours prior to enrollment.
2.1.2. Ethical Considerations
- Ethical approval was obtained from the Institutional Review Board (IRB) at each participating center and from the Republican Specialized Scientific and Practical Medical Center of Eye Microsurgery in Uzbekistan. Participants provided written informed consent after receiving a detailed explanation of the study’s objectives, procedures, and potential outcomes. The study complied with the ethical principles of the Declaration of Helsinki and adhered to Good Clinical Practice (GCP) guidelines to ensure the quality and validity of data.
2.1.3. Study Location and Relevance
- Uzbekistan was chosen as a study site due to its notable prevalence of adenoviral conjunctivitis and the limited availability of targeted treatments. This setting allowed the study to address a critical gap in the local management of Ad-Cj, contributing valuable data to global clinical practices.
2.1.4. Endpoints and Follow-Up
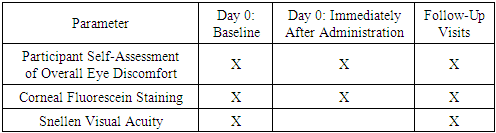
- The study’s primary endpoints included the reduction in clinical symptoms (e.g., redness, discomfort) and the safety profile of the interventions, assessed through fluorescein corneal staining, visual acuity testing, and participant-reported outcomes. These were evaluated at baseline, immediately post-application, and on Day 1. Secondary endpoints included the time to complete symptom resolution and the overall tolerability of treatments. The follow-up period extended to 7 days to capture short-term outcomes effectively.
2.2. Study Participants
- Patients were recruited from three clinical centers across Uzbekistan between March 2022 and July 2024. The inclusion criteria required participants to be 18 years of age or older and to have experienced symptoms of eye redness for no more than 4 days. During the screening process, participants underwent diagnostic confirmation through an adenoviral rapid test to ensure study eligibility. Those meeting all criteria were randomized into the study.
2.2.1. Exclusion Criteria
- Participants were excluded if they had a history of thyroid disorders (to minimize potential iodine-related complications), allergies to iodine or any study medications, recent ophthalmologic surgery (within the past 3 months), or any of the following ocular conditions: vesicular lesions, corneal dendrites, conjunctival membranes or pseudomembranes, subepithelial corneal infiltrates, corneal ulcers, injuries, or foreign bodies. Anterior chamber inflammation and pregnancy or lactation were also exclusion criteria to avoid confounding factors that could impact safety assessments.
2.2.2. Selection of Study Eye
- Only one eye per participant was included in the study. If both eyes were affected, the first symptomatic eye was selected for treatment. In cases where symptoms appeared simultaneously in both eyes, the study eye was determined randomly using a computer-generated sequence to maintain objectivity.
2.3. Study Protocol
- Before providing informed consent, participants were informed about the potential side effects of the 5% PVP-I solution, including mild irritation, a burning sensation, and temporary discoloration of the conjunctiva and eyelids. After confirming eligibility, patients underwent baseline assessments and were then randomized. Post-randomization, participants attended an initial visit and five follow-up visits on Days 1–2, Day 4 (Days 3–5), Day 7 (Days 6–10), Day 14 (Days 11–17), and Day 21 (Days 18–21).
2.3.1. Participant Surveys
- At each visit, participants completed a standardized 10-item questionnaire assessing eye discomfort and other symptoms. A trained physician or technician read the questions aloud and provided large-print response options on an A4 sheet for ease of use. Participants rated discomfort on a scale of 0 ("no discomfort") to 10 ("extreme discomfort"), capturing subjective tolerability outcomes.
2.3.2. Clinical Assessments
- Visual acuity (VA) testing was performed using a Snellen chart (with correction, without correction, or pinhole testing if VA was below 20/20). Corneal fluorescein staining (CFS) was graded using a scale from 0 (no staining) to 4 (dense staining) in five corneal sectors. The Brien Holden Vision Institute Visual Assessment System was utilized for consistency and reliability in grading [19].
2.3.3. Diagnostic and Molecular Testing
- To confirm adenoviral conjunctivitis, the study eye was anesthetized with one drop of 0.5% proparacaine solution. After 5 minutes, a rapid antibody test was performed according to the manufacturer's instructions [20]. Participants testing positive were randomized. Five minutes post-randomization, conjunctival swabs were collected for quantitative PCR (qPCR) testing for adenovirus detection. The swabs were stored at −80°C within four hours of collection and transported on dry ice to the central laboratory for molecular analysis, targeting specific adenoviral strains.
2.3.4. Purpose of Follow-Up Visits
- Each follow-up visit was designed to monitor treatment response, assess safety outcomes, and evaluate symptom progression. Adverse events, if reported, were documented and addressed immediately in accordance with the study's safety monitoring protocol.
2.3.5. Sample Size and Statistical Analysis
- The sample size was determined using a power analysis to ensure adequate power (80%) to detect statistically significant differences in the safety and tolerability endpoints with a significance level (α) of 0.05. In the absence of pilot data, the sample size calculation was based on findings from similar studies evaluating interventions for adenoviral conjunctivitis [3,5,7]. A clinically meaningful difference of 1.0 and a standard deviation of 1.5 were assumed, consistent with previous research on treatments for viral conjunctivitis [3,5,7]. The required sample size was calculated to be 30 participants per group. The calculation was performed using SAS software (version 9.4).Data analysis included descriptive statistics for baseline characteristics and inferential statistics for primary and secondary outcomes, with a focus on symptom resolution and tolerability.
2.4. Randomization
- Participants who met the inclusion criteria were randomized in a 1:1 ratio to receive either 5% povidone-iodine (PVP-I) solution or preservative-free artificial tears (AT). Randomization details were securely stored in numbered, sealed envelopes placed in coded boxes containing the assigned treatments. These boxes were distributed to the participating clinics by the Coordinating Center. Stratified randomization by clinic was conducted using a permuted block design with small block sizes to ensure balanced allocation across sites.A physician blinded to study outcomes administered the assigned treatment. First, the study eye was anesthetized with one drop of 0.5% proparacaine. Subsequently, 4–5 drops of the assigned solution (5% PVP-I or AT) were instilled. Participants were instructed to keep their eyes closed for 2 minutes, during which they moved their eyes in various directions. The physician gently pressed on the closed eyelids with a gloved finger to facilitate even distribution of the solution. The eyelid margins were then wiped with a gauze pad soaked in the assigned solution. After 2 minutes, the study eye was rinsed thoroughly with preservative-free buffered sterile saline, and the eyelid margins were cleaned again with a saline-soaked gauze pad to remove any remaining solution.
2.4.1. Post-Treatment Assessment and Follow-Up
- Immediately after receiving the treatment, participants rated their overall eye discomfort on a 0–10 scale, with 0 representing "no discomfort" and 10 representing "extreme discomfort."Participants were provided with detailed written instructions for infection control, including guidance on hand hygiene, avoiding touching or rubbing the eyes, and proper disposal of single-use artificial tears. Both study groups received artificial tears for home use, applied to the study eye 4 times daily for at least 5 days or until symptomatic improvement.Follow-up visits were conducted by physicians blinded to treatment allocation. At each visit, conjunctival swabs were collected for molecular analysis, and a rapid antibody test was performed until two consecutive negative results were obtained. Swabs were processed within four hours of collection and stored at −80°C before being sent on dry ice to the Coordinating Center for analysis.
2.4.2. Safety Monitoring
- Adverse events were monitored throughout the study. Any unexpected reactions were documented and addressed according to the study's safety protocol. Participants were instructed to report any severe or unusual symptoms immediately for further evaluation.
2.5. Safety and Tolerability
- The safety of 5% PVP-I was evaluated using corneal fluorescein staining (CFS), visual acuity (VA) testing, and monitoring for adverse events. CFS assessments were conducted at baseline, immediately after the administration of 5% PVP-I or artificial tears (AT), and on Day 1. The severity of staining was graded using the Brien Holden Vision Institute Visual Assessment System, which evaluates staining across five corneal sectors on a scale from 0 (none) to 4 (dense staining).Visual acuity was measured using a Snellen chart under standardized lighting conditions at baseline and reassessed on Day 1. Baseline values served as a reference to identify changes immediately after treatment and at the follow-up visit.Adverse events were actively monitored throughout the study, and any reported events were documented in detail, including their severity and duration. Participants were encouraged to report any unusual or severe symptoms immediately, and predefined criteria were used to grade and manage adverse reactions.Tolerability was assessed based on participants' self-reported overall eye discomfort, rated on a scale from 0 ("no discomfort") to 10 ("extreme discomfort"). These ratings were recorded at baseline, immediately after treatment, and on Day 1. Changes in discomfort levels over time were analyzed by comparing post-treatment and follow-up ratings to baseline values.Additional metrics, including patient-reported satisfaction and the timeline for symptom resolution, were collected as secondary measures to provide a comprehensive understanding of the intervention’s impact. Follow-up assessments extended beyond Day 1 for participants who reported lingering discomfort or adverse effects.
|
2.6. Statistical Methods
- Corneal fluorescein staining (CFS) scores from all five corneal sectors were summed to calculate a total CFS score [19]. Data are presented as mean ± standard deviation (SD). Visual acuity (VA) was assessed using a standardized Snellen chart and converted into the logarithm of the minimum angle of resolution (logMAR) for statistical analysis.Repeated measures analysis of variance (ANOVA) models were used to compare the two randomization groups across different time points. Separate ANOVA models were applied to each parameter: CFS, VA, and participants’ self-reported overall eye discomfort. The level of significance for all analyses was set at p < 0.05. Adjustments for multiple comparisons were made using the Bonferroni correction method to reduce the likelihood of type I errors.All statistical analyses were performed using SAS software version 9.4, chosen for its robust capabilities in handling repeated measures data and advanced modeling techniques.In total, 82% of participants attended their follow-up visits on Day 1 or Day 2. A subgroup analysis was conducted for participants who returned on Day 1 (n = 29) to evaluate any residual effects of the 5% PVP-I treatment. Participants who returned on Day 2 (n = 17) were included in the analysis based on data availability. Missing data were addressed by excluding cases with incomplete follow-up visits from specific analyses to maintain the validity of the results.The sample size was determined to provide sufficient power (80%) to detect clinically meaningful differences in the primary outcomes, defined as:Corneal Fluorescein Staining (CFS): A difference of at least 1 point in the total CFS score, indicating a significant change in corneal surface staining caused by the intervention.Visual Acuity (VA): A difference of 0.1 logMAR, reflecting a clinically relevant change in visual acuity.Discomfort: A difference of 2 points on the 0–10 discomfort scale, representing a meaningful change in patient-reported tolerability.These thresholds were selected based on clinical relevance and data from previous studies on adenoviral conjunctivitis treatments [3,5,7].
3. Results
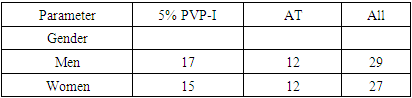
- Out of 212 patients screened at three clinical centers across Uzbekistan, 56 participants met the inclusion criteria and were randomized to receive a single application of either 5% PVP-I (n = 30) or preservative-free artificial tears (AT) (n = 26). The mean age of participants was 33.2 years ± 13.4 (Table 2). Both groups were balanced in terms of baseline demographics and clinical characteristics, including symptom duration and severity.
|
3.1. Corneal Fluorescein Staining
- In the 5% PVP-I group, the baseline total corneal fluorescein staining (CFS) score was 1.29 ± 2.0, which increased significantly to 3.25 ± 3.2 immediately after administration (p = 0.004). This transient increase in CFS likely reflects the mild irritation caused by the antiseptic properties of PVP-I. In the AT group, the baseline total CFS score was 1.79 ± 2.3, which slightly decreased to 1.65 ± 2.3 after administration; this change was not statistically significant (p = 0.79). The difference in changes from baseline to immediately after administration between the 5% PVP-I and AT groups was statistically significant (p = 0.03).On Day 1, the mean total CFS score in the 5% PVP-I group decreased to 1.65 ± 2.2, showing no significant difference compared to the baseline score (p = 0.63). Similarly, in the AT group, the mean total CFS score on Day 1 was 2.55 ± 2.2, which did not significantly differ from baseline (p = 0.26). No significant differences were observed between the two groups on Day 1 (p = 0.17) or in changes from baseline to Day 1 within each group (p = 0.26). Additionally, there were no significant differences in the direction of changes from baseline to Day 1 when comparing the two groups (p = 0.63).These findings suggest that while 5% PVP-I initially causes mild corneal irritation, this effect resolves quickly, with no long-term adverse effects on the corneal surface.
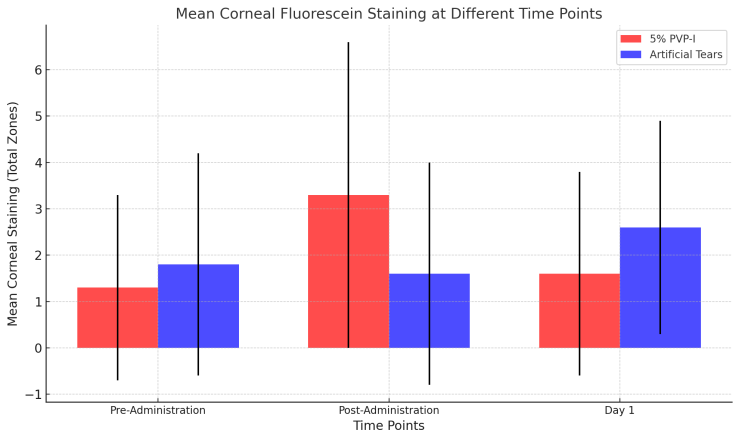 | Figure 1. Mean Corneal Fluorescein Staining at Different Time Points |
3.2. Visual Acuity
- In the 5% PVP-I group, baseline visual acuity, measured using the logarithm of the minimum angle of resolution (logMAR), was 0.09 ± 0.12. This improved slightly to 0.08 ± 0.15 on Day 1, but the change was not statistically significant (p = 0.88). Similarly, in the AT group, baseline visual acuity was 0.12 ± 0.28 logMAR, improving to 0.09 ± 0.11 by Day 1, with no significant change (p = 0.72).No statistically significant differences were observed between the two groups (p = 0.68), across time points (p = 0.72), or in the interaction between group assignment and time points from baseline to Day 1 (interaction p = 0.88).While the observed improvements in visual acuity were not significant, they may reflect natural symptom resolution or minor benefits of treatment. LogMAR scores, where lower values indicate better visual acuity, provide a standardized way to compare outcomes. Visual acuity assessments beyond Day 1 could help clarify whether these trends persist over time.
3.3. Adverse Events
- During the 21-day follow-up period, no adverse events were reported in the AT group. In the 5% PVP-I group, one adverse event was recorded during the Day 1 visit: "photophobia with a mild anterior chamber reaction." The event was evaluated by a masked physician, who classified it as "unrelated to the treatment" based on predefined criteria for causality assessment.The participant continued with the study without requiring additional interventions, and the symptoms resolved spontaneously by the next scheduled visit. While photophobia and mild anterior chamber reactions are rare, they are recognized potential side effects of ocular antiseptics like PVP-I, emphasizing the importance of careful monitoring in such studies.
3.4. Participants’ Self-Assessment of Overall Eye Discomfort
- At the baseline visit, participants rated their overall discomfort in the study eye (n = 56) on a scale from 0 ("no discomfort at all") to 10 ("extreme discomfort"). The mean baseline discomfort scores were similar between the 5% PVP-I group (6.1 ± 3.1) and the AT group (6.8 ± 2.5), with no statistically significant difference (p = 0.34).In the 5% PVP-I group, the discomfort level immediately after treatment (6.2 ± 2.8) did not differ significantly from the baseline level (6.0 ± 3.0, p = 0.78). Conversely, in the AT group, the discomfort level immediately after treatment (3.2 ± 2.7) was significantly lower than the baseline level (6.7 ± 2.6, p < 0.0001). The marked reduction in discomfort in the AT group could be attributed to the soothing and lubricating properties of artificial tears, which provide immediate relief for ocular irritation.The difference in the change in discomfort levels between the 5% PVP-I and AT groups from baseline to immediately after treatment was statistically significant (p = 0.0013). This suggests that the initial tolerability of PVP-I may be lower due to its antiseptic properties, which could cause transient irritation.On Day 1, participants’ discomfort levels in both groups showed no significant changes compared to baseline (p = 0.10). Moreover, there were no significant differences between the two groups on Day 1 (p = 0.22) or in the direction of changes from baseline between the groups (p = 0.83). These findings indicate that while the immediate tolerability of 5% PVP-I may be lower, it does not lead to sustained discomfort over time.This data highlights the importance of counseling patients about the potential for transient discomfort with PVP-I, balanced by its potential efficacy against adenoviral conjunctivitis.
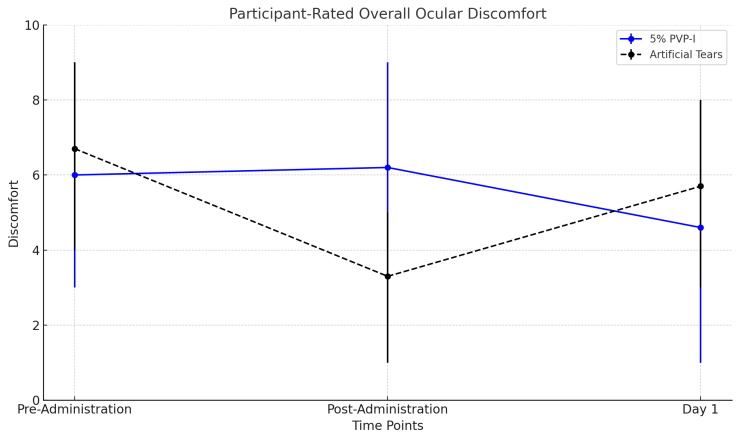 | Figure 2. Participant-rated ocular discomfort |
4. Discussion
- Povidone-iodine (PVP-I) has long been established as a safe and effective ophthalmic antiseptic in surgical procedures. However, in vivo studies examining its tolerability from the patient's perspective are relatively scarce. In a retrospective study of 1,854 patients receiving intravitreal injections, Peden et al. found that 16% of patients reported sensitivity to standard ophthalmic 5% PVP-I [21]. Similarly, Saedon et al. reported increased symptoms of dry eye and corneal epithelial staining in eyes subjected to repeated intravitreal injections after antisepsis with 5% PVP-I. The comparison was made with the patients’ contralateral eyes, which had not undergone injections or PVP-I exposure, highlighting the potential for cumulative effects [22].Ridder et al., in a smaller study of 10 participants, compared the effects of a 2-minute exposure to 5% PVP-I versus artificial tears (AT) on symptoms of dry eye, corneal staining, and visual acuity. While AT caused minimal signs and symptoms, 5% PVP-I exposure resulted in a transient increase in corneal staining, a temporary reduction in visual acuity, and exacerbated dry eye symptoms. By 24 hours, no differences were observed between the AT-treated and PVP-I-treated eyes in terms of visual acuity and dry eye symptoms, but mild corneal staining persisted in the PVP-I group [23].Previous research primarily evaluated the safety and tolerability of PVP-I in eyes without significant anterior segment pathology, focusing on various concentrations and exposure durations. In contrast, the current study specifically investigated the safety and tolerability of a single 2-minute exposure to ophthalmic 5% PVP-I in eyes with presumed adenoviral conjunctivitis (Ad-Cj). This is a critical distinction, as the pathological state of the ocular surface in active Ad-Cj may reduce tolerability to PVP-I.The findings of this study not only highlight the transient nature of adverse effects associated with PVP-I but also provide a foundation for understanding its application in managing viral conjunctivitis. Future research should explore the long-term impact of PVP-I on ocular surface health, its efficacy compared to other antiseptic agents, and strategies to improve tolerability in patients with compromised ocular surfaces.
4.1. Safety and Tolerability
- Safety and tolerability were evaluated through corneal fluorescein staining (CFS), visual acuity (VA) measurements, monitoring of adverse events, and participants' self-assessment of discomfort at baseline, immediately after treatment, and on Day 1. Toxic effects from ophthalmic solutions are typically transient and resolve within 24 hours. Therefore, while the study protocol allowed the first follow-up visit within 24–48 hours after baseline, this analysis focuses on the subgroup of participants who returned for follow-up on Day 1 to capture acute effects.The study observed an increase in CFS immediately after administering 5% PVP-I. However, by Day 1, CFS scores returned to baseline, suggesting that the transient corneal staining was likely due to the antiseptic’s temporary irritation. This rapid recovery can be attributed to thorough rinsing with saline after the 2-minute exposure and the participants' use of preservative-free artificial tears (AT) four times daily. These findings reinforce the importance of post-treatment rinsing and supportive care in mitigating short-term ocular irritation.In the AT group, a significant reduction in discomfort was reported immediately after administration. This demonstrates the soothing benefits of ophthalmic moisturizers in managing adenoviral conjunctivitis (Ad-Cj), either as standalone treatments or as adjuncts to other therapies. Corneal staining levels on Day 1 were minimal and aligned with those typically observed in successful daily and extended wear contact lens users, supporting the safety profile of these interventions [24].
4.2. Visual Acuity and Symptoms
- Participants maintained stable visual acuity throughout the study, and reported symptoms were minimal. These results confirm the safety of 5% PVP-I for patients with presumed Ad-Cj. A slight increase in mean CFS scores in the AT group from 1.8 at baseline to 2.6 on Day 1 likely reflects the natural progression of Ad-Cj rather than treatment-related effects. Importantly, this increase did not result in any reduction in visual acuity, indicating that it had no functional impact.
4.3. Comparison with Prior Research
- A Phase 2 randomized trial conducted by Pepose et al. investigated the safety and tolerability of 0.6% PVP-I combined with 0.1% dexamethasone, 0.6% PVP-I alone, and a control solution in patients with Ad-Cj. Participants rated their comfort during administration and at one- and two-minutes post-administration using a 0–10 scale (0 = very comfortable, 10 = very uncomfortable). Comfort levels were comparable across all groups, with mean scores ranging from 2 to 3, demonstrating similar tolerability. However, data on comfort during subsequent visits were not reported. The authors highlighted that comparable comfort between the control and study treatments is crucial, as patient adherence depends heavily on perceived comfort.The study by Pepose et al. did not identify safety concerns based on VA assessments or slit-lamp biomicroscopy. Among participants, 281 treatment-related adverse events were reported in 61.7% of cases. All events were mild or moderate and deemed unrelated to the study treatments. The combination therapy is now undergoing Phase 3 trials, which may provide further insights into long-term safety and efficacy [16].
4.4. Clinical Implications and Future Directions
- The findings of this study and prior research collectively suggest that 5% PVP-I is a safe option for treating Ad-Cj, with transient discomfort as the main tolerability issue. The significant reduction in discomfort observed with AT suggests that combining PVP-I with artificial tears or corticosteroids could enhance patient comfort and adherence. Future studies should explore these combinations over extended follow-up periods to assess long-term safety and efficacy. Additionally, examining patient-reported outcomes, such as quality of life and treatment satisfaction, could further inform clinical practices.
5. Conclusions
- In conclusion, our study confirms that ophthalmic 5% PVP-I is a safe and well-tolerated treatment for patients with presumed adenoviral conjunctivitis (Ad-Cj). Although a transient increase in corneal staining may occur, the associated discomfort can be effectively mitigated by the regular use of artificial tears. Educating patients about the short-lived nature of these effects can enhance treatment acceptance and adherence, reassuring those presenting with "red eye" symptoms.These findings provide clinicians with evidence-based support for confidently incorporating 5% PVP-I into the management of Ad-Cj. With no significant concerns regarding safety and tolerability, this treatment approach represents a valuable addition to current therapeutic options. Further research could explore combining PVP-I with other agents, such as corticosteroids, to enhance both efficacy and patient comfort.
ACKNOWLEDGEMENTS
- We extend our sincere gratitude to all the participants who volunteered for this study, as their contribution was invaluable to advancing our understanding of 5% PVP-I in the management of adenoviral conjunctivitis. We are also deeply appreciative of the clinicians, research staff, and coordinators at the participating clinical centers in Uzbekistan for their dedication and support throughout the study.We acknowledge the Republican Specialized Scientific and Practical Medical Center of Eye Microsurgery in Uzbekistan for their guidance and for providing the infrastructure necessary to conduct this research. Special thanks to the Coordinating Center for their logistical and analytical assistance.Finally, we express our gratitude to our colleagues and collaborators for their insightful feedback and contributions to the study design, execution, and interpretation. This work would not have been possible without their collective effort and expertise.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML