-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(12): 3425-3429
doi:10.5923/j.ajmms.20241412.74
Received: Dec. 12, 2024; Accepted: Dec. 26, 2024; Published: Dec. 31, 2024

Clinical Manifestations of Papillomavirus Infection in Women with Secondary Infertility
L. R. Agababyan, Z. Sh. Israilova
Department Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article analyzes the main factors of female infertility, including tubal-peritoneal, endocrine, immunological, infertility caused by genital diseases, and idiopathic infertility. The etiological aspects are considered, including the influence of infectious agents on female fertility. Particular attention is paid to the role of sexually transmitted infections - the human papillomavirus, in the development of infertility. Data from numerous studies on the prevalence of HPV and its impact on women's reproductive health are presented. The article emphasizes the importance of a comprehensive approach to the diagnosis and treatment of female infertility to improve treatment outcomes and increase the chances of successful conception.
Keywords: Secondary infertility, Papillomavirus infection, Condyloma acuminata, Diagnosis of "thin" endometrium
Cite this paper: L. R. Agababyan, Z. Sh. Israilova, Clinical Manifestations of Papillomavirus Infection in Women with Secondary Infertility, American Journal of Medicine and Medical Sciences, Vol. 14 No. 12, 2024, pp. 3425-3429. doi: 10.5923/j.ajmms.20241412.74.
1. Introduction
- In the field of reproductive health today, infertility occupies a special place. According to modern sources, 8 to 12% of married couples have infertile marriages, in which the woman's share is about 50-80% [3,6,10,29,38].There are five main groups of factors of female infertility:Tubal-peritonealEndocrineInfertility caused by genital diseases and anatomical abnormalitiesImmunologicalIdiopathic (infertility of unknown origin)Analyzing these factors, it should be recognized that infectious agents can become a predictor of female infertility in all five groups. Undoubtedly, infection affects the condition of the fallopian tubes and endometrium, but the inflammatory autoimmune process contributes to the disruption of the structure and function of the ovaries. And finally, the disruption of the vaginal microbiota in connection with the presence of microorganisms with impaired immunoreactivity contributes to the development of antisperm antibodies and impaired sperm motility [7,9,8,11,12,23,14,31,24,34]. In this regard, the problem of sexually transmitted infections (chlamydia, ureaplasmosis, mycoplasmosis, gonorrhea), as predictors of infertility, has already been sufficiently studied. However, the etiological role of viral sexually transmitted infections (STIs) in fertility impairment, including human papillomavirus infection, has not yet been studied. The frequency of human papillomavirus infections (HPV) in the world, according to various researchers, has increased more than 10 times over the past decade [5,4,2,20,21,16,22,35,36,27]. Almost all of the reports cited are based on the detection and treatment of oncogenic HPV strains. However, in practice, clinical manifestations of the human papillomavirus in the form of condyloma acuminata are increasingly encountered, which is the main reason for women to seek medical attention, in whom, upon closer examination, other infections are also detected [19,17,37,40]. This state of affairs even led to the need to publish clinical guidelines for the management of women with condyloma acuminata [32]. Although most researchers agree that human papillomavirus infection itself does not lead to infertility in women, but can be the cause of male infertility in her partner [5,25,26,30,18,39]. the frequent referral of infertile patients with manifestations of condyloma acuminata served as the reason for conducting this study.Objective of the study: To study the condition of the endometrium in infertile patients with condyloma acuminata.
2. Materials and Methods
- Using ultrasound, the condition of the endometrium was studied in 78 patients with secondary infertility who applied to the private medical clinic MCHJ "Mama i Ya" in 2022-2023 (main group). The control group consisted of 50 patients with secondary infertility without condyloma acuminata.The inclusion criteria for the study were: age not older than 35 years; absence of developmental anomalies of the pelvic organs; history of pregnancy; absence of pregnancy for 12 months without the use of contraceptives; signed informed consent to participate in the study; condyloma acuminata detected during genital examination.Exclusion criteria: age over 35 years; absence of condyloma; congenital malformations of the pelvic organs; somatic diseases that are contraindications for pregnancy and childbirth; patient's refusal to participate in the study; alcohol abuse, drug or drug addiction; male infertility.Standard clinical and gynecological research methods were used. In the middle of the menstrual cycle, an ultrasound examination of the pelvic organs with Dopplerography was performed on a Sonoscape 3D/4D device.The obtained results were subjected to statistical processing using methods presented in the Statistica 6.0 program. To assess the differences between groups according to quantitatively measured indicators, the parametric Student's t-test was used. To compare the control and main groups according to qualitative characteristics, the Fisher's multifunction criterion was used. Using the χ2 criterion, the frequency of Dopplerometry changes in the groups was compared. Statistically significant differences were considered at p<0.05 (95% confidence level).
3. Results
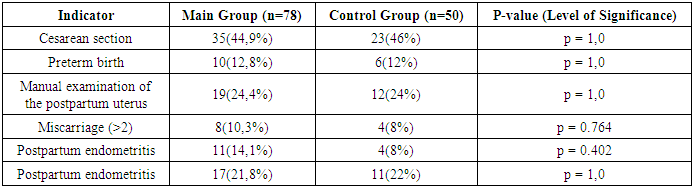
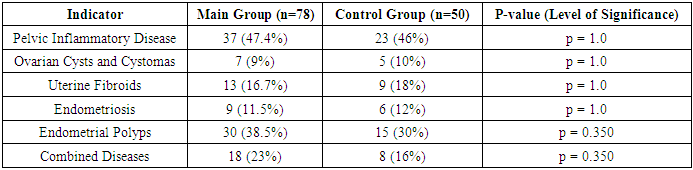
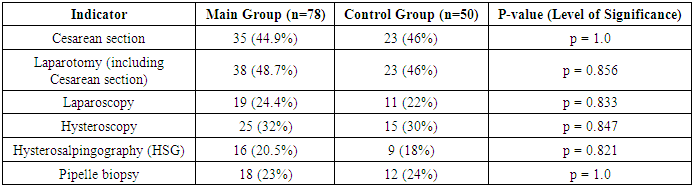
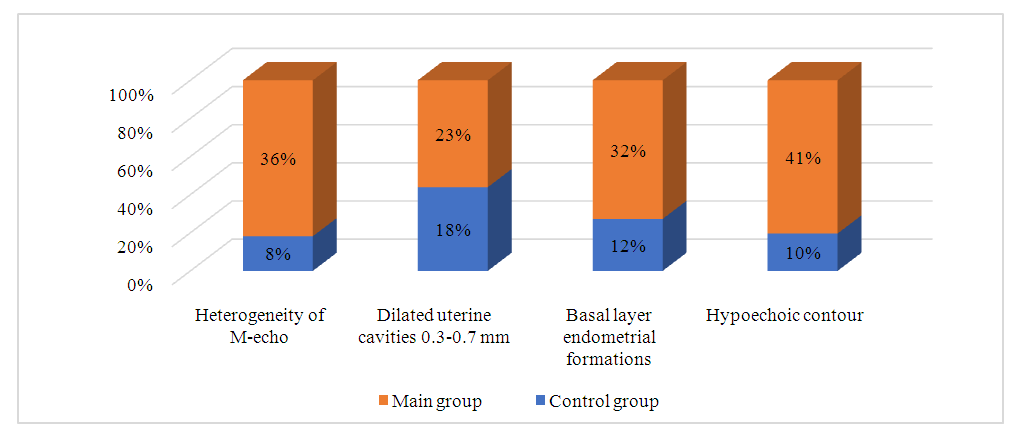
- The patients included in the study were aged 25 to 35 years. We did not find statistically significant differences in age between the study groups: the average age of patients in the main group was 30.5±1.2 years, patients in the control group – 30.2±1.29. When comparing the main characteristics of the menstrual cycle according to anamnestic data (menarche, duration of the menstrual cycle, duration and nature of menstruation), it was shown that the groups are comparable in all these parameters. There were no significant differences in marital status and education level between the patients in both groups.Of the somatic diseases, anemia, which was found with the same frequency in both groups of women – 50% and 52% respectively, is noteworthy. Mostly it was chronic iron deficiency anemia of moderate severity.Particular attention was paid to the obstetric history and gynecological morbidity in the examined women, as well as to the types of surgical interventions performed on the pelvic organs (Tables 1, 2, 3).
|
|
|
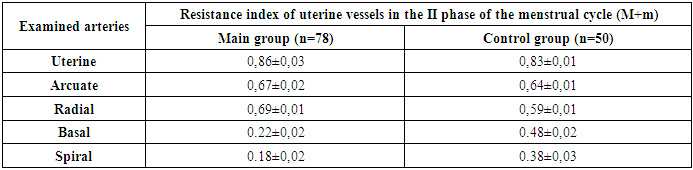
 | Figure 1. Dopplerometry |
|
4. Discussion
- Analyzing the data we obtained, we can argue that HPV-positive patients have prerequisites for the development of infertility. This is also evidenced by studies conducted by Yu.N. Banashkaeva and co-authors (2022), which indicate that even in the treatment of infertility using assisted reproductive technologies, the lowest frequency of pregnancy after cryoprotocols of IVF was observed in the group of HPV-positive women (33.3%) compared to HPV-negative (73.9%) and previously vaccinated patients (60.7%); p<0.05 [1]. The work of Italian researchers showed that in HPV-positive patients, unsuccessful IVF attempts were almost 3 times more common compared to HPV-negative (40 and 13.5%) [28]. According to the data of the US university clinic, when conducting ART programs in women with HPV persistence, the frequency of pregnancy was significantly lower than in women without the virus (57 and 23.5%) [15].The negative impact of HPV on reproductive function is also confirmed in a number of other works. Thus, a systematic review conducted in Germany demonstrates a reliable association between spontaneous abortion, spontaneous preterm birth and the presence of HPV both in the cervix and in the placenta [13].
5. Conclusions
- The combination of condyloma acuminata with "thin" endometrium in patients with secondary infertility requires further research and determination of the tactics for managing these women.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML