-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(12): 3398-3401
doi:10.5923/j.ajmms.20241412.68
Received: Dec. 11, 2024; Accepted: Dec. 22, 2024; Published: Dec. 31, 2024

The Effect of Calcium Homeostasis and Immune Disorders on the Development of Eczema: A Pathomorphological Study
Nasritdinova N. B.1, Sidikov A. A.2
1Andijan State Medical Institute, Andijan, Uzbekistan
2Rector, Fergana Medical Institute of Public Health, Fergana, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Eczema, or atopic dermatitis, is a chronic inflammatory skin disorder characterized by disrupted skin barrier function, dryness, and recurrent episodes of pruritus and inflammation. While the pathogenesis of eczema is multifactorial, increasing evidence suggests that dysregulated calcium homeostasis in keratinocytes and aberrant immune responses play significant roles. Calcium ions serve as critical regulators of epidermal differentiation, barrier formation, and cell-to-cell adhesion. Concurrently, immune system imbalances—including T-helper type 2 (Th2)-driven inflammation, reduced regulatory T-cell function, and altered cytokine signaling—can exacerbate skin barrier defects and inflammatory responses. This article synthesizes current knowledge on the interplay between calcium signaling pathways, immune dysregulation, and their combined effect on eczema development. By examining these mechanisms, we highlight potential avenues for targeted therapeutic strategies aimed at restoring calcium equilibrium, modulating immune responses, and ultimately improving clinical outcomes for patients with eczema.
Keywords: Eczema, Atopic Dermatitis, Calcium Homeostasis, Immune Disorders, Skin Barrier, Th2 Inflammation
Cite this paper: Nasritdinova N. B., Sidikov A. A., The Effect of Calcium Homeostasis and Immune Disorders on the Development of Eczema: A Pathomorphological Study, American Journal of Medicine and Medical Sciences, Vol. 14 No. 12, 2024, pp. 3398-3401. doi: 10.5923/j.ajmms.20241412.68.
Article Outline
1. Introduction
- Eczema in the elderly population continues to rise and has become a major public health problem. The pathogenesis of eczema is not yet clear, but the research we have so far shows that calcium homeostasis disorders, immune dysfunction, and allergies all play a role in the development of eczema. However, there are few reports on the relationship between calcium homeostasis disorders and immune function in eczema. This article will review the available literature and present an overview of how calcium homeostasis and immune disorders affect the development of eczema. Eczema is a common skin disorder that often occurs in the first few months of life and is a heterogeneous group of skin diseases including, among others, atopic dermatitis and contact dermatitis. The number of elderly patients with eczema is increasing year by year, and the pathogenesis has been extensively studied with reports showing a close relationship between calcium homeostasis, immune regulation, and the occurrence and development of eczema. Calcium is the most important intracellular second messenger. Various hormones, growth factors, cytokines, chemokines, integrins, and neurotransmitters all regulate cytoplasmic calcium levels through calcium channels, pumps, exchangers, and release from organelles. Calcium therefore regulates various cell processes such as differentiation, growth, motility, secretion, phagocytosis, and apoptosis. A large number of reports show that calcium homeostasis plays an important role in the process of inflammation and immune regulation; that is, a decrease in intracellular calcium leads to a decrease in the number of T cells and B cells and a downregulation in the immune response. As the age of the elderly increases, the lack of physical mobility leads to a decrease in the number of Langerhans cells and a decrease in the secretion of proinflammatory factors. These changes, in turn, directly affect the therapeutic effect of patients with eczema and other inflammatory immune-related skin diseases. In this paper, we present a review of research on calcium and its distribution, immunity, and role in the pathogenesis and treatment of eczema in order to understand more fully the important role of calcium in the eczema process.Background and RationaleEczema is an inflammatory skin disorder with high prevalence in childhood. Eczema can lead to a negative impact on the patient, the family, and society. Patients suffer from secondary skin bacterial infections, sleep disturbances, lower grades at school, and psychological distress, especially mental health comorbidities and compensation. The direct medical costs were significantly higher in eczema patients than in matched non-eczema patients. The indirect costs, including the loss of parental productivity, presenteeism, care for the child with eczema, and general practitioner contacts, were doubled. Therefore, understanding the pathogenesis and developing a biologic therapy is attractive.The dysregulated immune response with an imbalanced T helper 1 (Th1) and T helper 2 (Th2) lymphocyte response to antigens could contribute to a broad range of allergic diseases, including eczema. In recent years, immunological pathways, including the interaction with dendritic cells, macrophages, eosinophils, and keratinocytes, have been validated with substantial evidence. However, treating the patient with a systemic immunosuppressive agent is an old approach that could induce a secondary infection and even multiple organ failure. Furthermore, previous studies revealed that transplanting immune response cells regulated by IL-10 signaling promoted wound healing, not a decrease in the severity of chronic illness via suppression of the immune system. How can we develop a new way to modulate the excessive immune response? It is an emerging issue.Literature ReviewEarly studies on eczema’s pathophysiology largely emphasized the structural and biochemical elements of the skin barrier. Palmer et al. (2006) [1] demonstrated that loss-of-function variants in filaggrin, a key structural protein, were significantly associated with atopic dermatitis, thus focusing initial attention on genetic and biochemical factors underlying barrier defects. However, more recent research has moved beyond static structural considerations to include dynamic ionic and immunological mechanisms. Menon (2002) [2] highlighted that normal epidermal calcium gradients—high in the stratum granulosum and lower in the basal layers—are essential for keratinocyte differentiation and lamellar body secretion. Similarly, Elias and Schmuth (2009) [3] presented evidence that disturbances in these gradients undermine corneocyte structure, lipid organization, and thereby barrier stability.Building on these findings, Hennings et al. (1980) [4] pioneered studies on how calcium concentrations affect keratinocyte proliferation and differentiation in vitro, showing that carefully modulated calcium levels optimize epidermal tissue formation. Disrupted calcium homeostasis, therefore, not only impedes the generation of a robust barrier but can also predispose skin to irritation and allergen entry.In parallel, Leung and Guttman-Yassky (2014) [5] advanced the understanding of immune dysregulation in atopic dermatitis, describing how a skewed T-helper type 2 (Th2)-dominant response drives the production of cytokines—such as IL-4, IL-13, and IL-31—that weaken the skin barrier and promote itching and inflammation. Kim et al. (2008) [6] expanded on this mechanism by demonstrating that Th2 cytokines suppress the expression of essential structural proteins like loricrin and involucrin, further compounding barrier fragility. Belkaid and Segre (2014) [7] added another dimension to this immunological perspective, illustrating how disrupted immune homeostasis affects the skin microbiome, increasing susceptibility to infections and perpetuating inflammatory cycles.Additionally, research by Denda et al. (2007) [8] and Mauro et al. (1998) [9] delved into the interplay between calcium signaling and immune factors. These studies suggest that altered calcium gradients influence keratinocyte-derived cytokine and lipid profiles, thereby shaping local immune environments within the epidermis. This bidirectional relationship implies that not only does immune imbalance affect barrier integrity, but calcium-sensitive pathways within keratinocytes can also modulate inflammatory cell infiltration and activation.
2. Materials and Methods
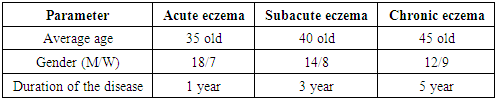
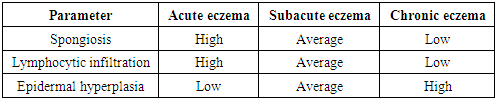
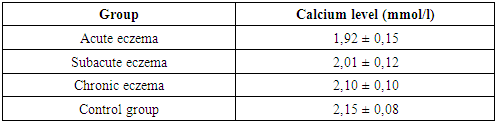
- The study was conducted on a sample of 45 patients with various forms of eczema (acute, subacute, chronic). All patients underwent skin biopsies with subsequent morphological and immunohistochemical analysis. Antibodies against CLA-HECA-452, CD1a and CD207/Langerin were used to assess the activity of Langerhans cells and other immune cells. In addition, serum calcium levels were measured in patients to study the correlation with the severity of the disease.
3. Analysis and Results
|
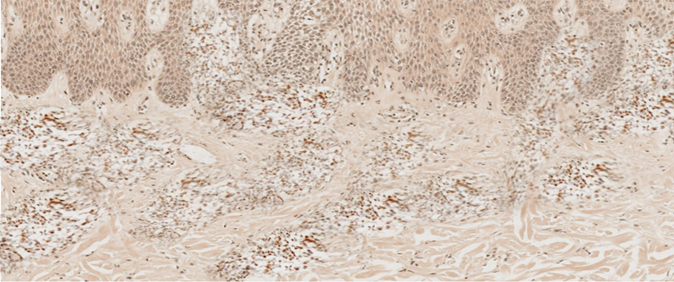 | Figure 1. Diagnosis of "discoid eczema", chronic form. Both in the papillary and in the reticular layers of the dermis, there is a pronounced expression of the marker CLA-(HECO-452). IGH. ×100 |
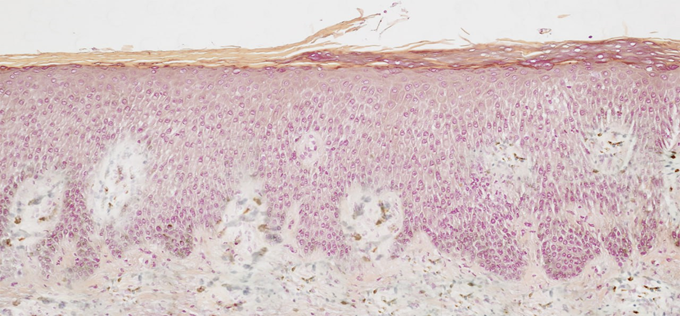 | Figure 2. Diagnosis: "discoid eczema", chronic form. In the epidermis, a weak reaction of the CD1a+ antigen is noted. IHC. ×400 |
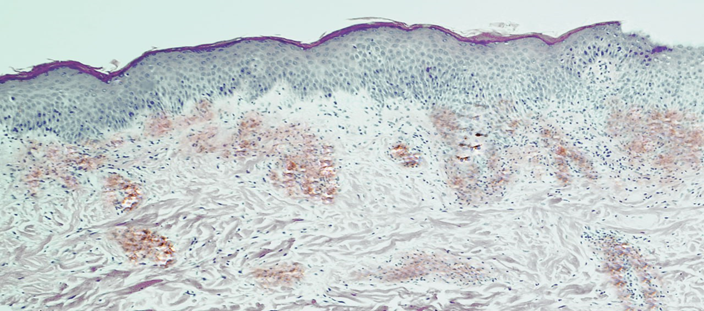 | Figure 3. Diagnosis: "discoid eczema", subacute form. In the dermis - a sharply expressed expression of the CD207/Langerin marker. IHC. ×100 |
|
|
4. Discussion
- The results of the study confirm that morphological changes in the skin in eczema are directly related to the phase of the disease. In the acute stage, pronounced inflammatory processes dominate, which weaken during the transition to the chronic stage. Immunohistochemical analysis shows that the activation of Langerhans cells and T cells plays a key role in the initial stages of inflammation, while their activity decreases as the process becomes chronic.The level of calcium in the blood serum is also an important indicator of the severity of the disease. In patients with acute eczema, a significant decrease in calcium levels is observed, which may indicate a violation of calcium homeostasis and its role in the pathogenesis of eczema.
5. Conclusions
- The interplay between calcium homeostasis and immune dysfunction significantly contributes to the development and persistence of eczema. Disruptions in epidermal calcium gradients compromise the proper differentiation of keratinocytes, leading to a weakened skin barrier more susceptible to irritants, allergens, and pathogens. In parallel, immune imbalances—particularly Th2-driven responses and reduced regulatory control—aggravate inflammation, dampen the expression of essential structural proteins, and inhibit repair mechanisms, thereby perpetuating the cycle of barrier disruption and chronic inflammation. Recognizing that eczema pathogenesis emerges from both ionic signaling disturbances and immunological dysregulation underscores the need for integrative therapeutic strategies. Approaches that restore normal calcium dynamics may improve barrier integrity and resilience, potentially enhancing the efficacy of existing anti-inflammatory therapies. Similarly, treatments targeting key immunological pathways can alleviate the inflammatory burden, allowing the barrier to recover more efficiently when calcium homeostasis is supported. Future research should focus on elucidating the precise molecular links between calcium signaling and immune modulation, as well as exploring combination therapies that address both dimensions simultaneously. By refining our understanding and intervention methods, it may be possible to break the vicious cycle of damage and inflammation, offering patients with eczema more durable relief and improved quality of life.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML