-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(12): 3141-3148
doi:10.5923/j.ajmms.20241412.12
Received: Nov. 20, 2024; Accepted: Dec. 8, 2024; Published: Dec. 10, 2024

Combination of Volumetric Angiography and Two-dimensional Dopplerometry: Diagnostic Informativity in Differential Diagnostics of Endometrial Pathology in Menopausal Women
Lorida Erkinovna Shamsiyeva
Center for the Development of Professional Qualification of Medical Workers, Tashkent, Uzbekistan
Correspondence to: Lorida Erkinovna Shamsiyeva, Center for the Development of Professional Qualification of Medical Workers, Tashkent, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to evaluate the diagnostic informativity of the combined study in the modes of three-dimensional angiography and two-dimensional Dopplerography with the use of uterine arterial perfusion indices in the differential diagnostics of endometrial hyperplastic processes and prediction of endometrial malignant pathology in women with abnormal uterine bleeding at the age of menopause. Background. The hormonal disorders affecting all links of neurohumoral regulation of a woman's body lead to pathological transformation of the endometrium. Endometrial cancer occupies the 3rd place in the structure of cancer morbidity in the female population, according to statistical studies in Uzbekistan. Based on the data of scientific studies of foreign authors, only 4% of patients with endometrial carcinoma are under 40 years. It is usually diagnosed in postmenopausal women. Material and Methods. From April to September 2024, 98 women admitted to the Tashkent regional branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology with complaints of uterine bleeding against the background of menopause lasting 5-15 years were examined. The women's ages ranged from 51 to 72 years (mean age was 61.5 years). All examined patients (n=98) were divided into two groups. Results. A slight increase of blood flow in uterine arteries was observed in Group I patients with benign endometrial hyperplasia. Group II patients with endometrial adenocarcinoma had increased subendometrial and intraendometrial blood flow due to the appearance of neoangiogenesis. Volumetric blood flow indices and RI were evaluated and compared with tumor stage and grading, myometrial infiltration along with metastasis to lymph nodes. A significant improvement in sensitivity and specificity was found in the differentiation of endometrial hyperplasia and endometrial carcinoma when 3D transvaginal angiography and energy Doppler imaging were combined. Conclusion. The obtained results indicate the high efficiency of the combination of methods of qualitative and quantitative assessment of blood flow in two-dimensional and three-dimensional modes for revealing the characteristic features of malignant transformation, which plays an important role in the clarifying diagnostics and choice of treatment tactics.
Keywords: Endometrial hyperplasia, Endometrial cancer, Doppler ultrasound of endometrial vessels, Volumetric angiography, Vascularization index, Uterine arterial perfusion index, Volumetric blood flow
Cite this paper: Lorida Erkinovna Shamsiyeva, Combination of Volumetric Angiography and Two-dimensional Dopplerometry: Diagnostic Informativity in Differential Diagnostics of Endometrial Pathology in Menopausal Women, American Journal of Medicine and Medical Sciences, Vol. 14 No. 12, 2024, pp. 3141-3148. doi: 10.5923/j.ajmms.20241412.12.
Article Outline
1. Introduction
- Hyperplastic processes in the endometrium represent a major biomedical and socioeconomic problem and occupy a significant place in the structure of gynecologic morbidity [1-6]. The hormonal disorders affecting all links of neurohumoral regulation of a woman's body lead to pathological transformation of the endometrium. The endometrium is a target organ for sex hormones due to the presence of specific receptors in it. Balanced hormonal impact provides physiological cyclic transformations of the uterine mucosa. Disorder of hormonal homeostasis of a woman can lead to changes in the growth and differentiation of endometrial cellular elements and entail the development of hyperplastic or neoplastic processes in the endometrium. Hyperestrogenism plays a leading role in pathogenesis. Endometrial cancer (EC) risk factors, in addition to the age of the patient are obesity, late menopause, diabetes mellitus and taking tamoxifen. Obesity and a body mass index (BMI) over 35.0 increases the risk of developing EC by 200-400%. As more than 50% of postmenopausal women are overweight, cancer alertness in this age group is necessary [7-9]. The great clinical significance of hyperplastic processes in the endometrium is that they are one of the most common causes of uterine bleeding in women aged 45-55 years and their hospitalization [10-11].Endometrial cancer occupies the 3rd place in the structure of cancer morbidity in the female population, according to statistical studies in Uzbekistan. Endometrial polyps are found in 5.3-25% of gynecologic patients of all age groups, but most commonly in pre- and postmenopausal women. Endometrial hyperplasia, especially when atypia is present, is a serious clinical problem because it may precede malignant endometrial lesions. Assessment of endometrial health has changed over the past few years with the recognition that endometrial pathology is not always global in nature [1-3]. The high probability of malignization of hyperplastic processes in the endometrium, as well as the lack of proper efficiency of hormonal therapy, put endometrial hyperplasia (EH) among the most urgent problems of modern medicine. Malignization of glandular hyperplasia and endometrial polyp occurs in 2-5% and reaches 10-15% at postmenopausal age. Typically, endometrial cancer (EC) is clinically manifested by uterine bleeding, although identical clinical manifestations occur in benign conditions, with a number of endometrial biopsies being performed unnecessarily. The diagnosis of endometrial carcinoma in young women of childbearing age is rare. According to scientific research by foreign authors, only 4% of patients with endometrial carcinoma are under 40 years of age. It is usually diagnosed in postmenopausal women [4]. EC by histologic examination is confirmed in 1-10% of postmenopausal women with bleeding. Endometrial atrophy is detected in almost 60% of observations and endometrial polyps, EH and other benign endometrial changes are detected with approximately equal frequency [5-6]. An accurate non-invasive diagnostic test is needed to evaluate the nature of the endometrial hyperplastic process in order to limit inappropriate surgical intervention as much as possible. Echography in volumetric mode has entered clinical practice relatively recently. Its main advantages include: the ability to obtain ultrasound slices that are not available with conventional seroscale B-mode scanning and to make accurate measurements of voluminous endometrial masses. According to studies by W.P. Martins (2007) and L.T. Merce, volumetric values such as flow index and vascularization index established by three-dimensional echography have higher accuracy and reproducibility. The aim of the study was to evaluate the diagnostic informativity of the combined study in the modes of three-dimensional angiography and two-dimensional Dopplerography with the use of uterine arterial perfusion indices in the differential diagnostics of endometrial hyperplastic processes and prediction of endometrial malignant pathology in women with abnormal uterine bleeding at the age of menopause.
2. Material and Methods
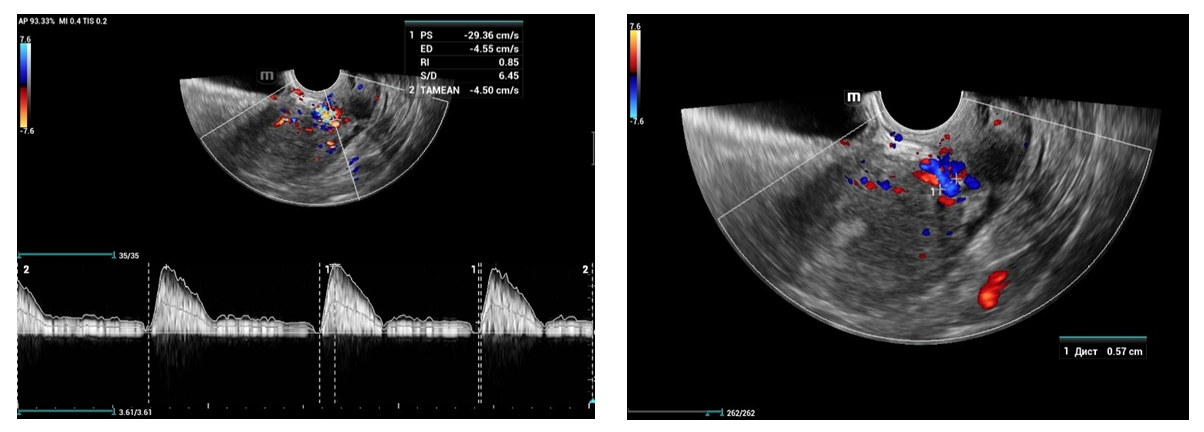
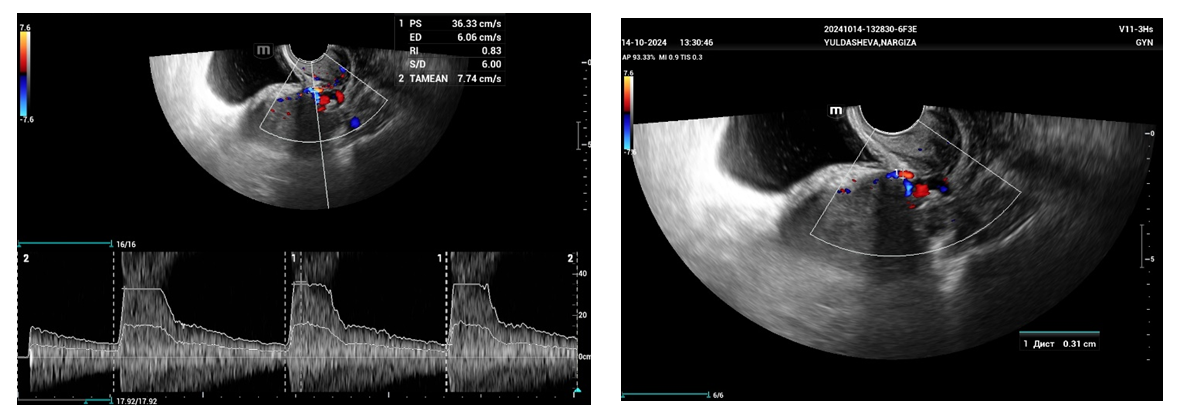
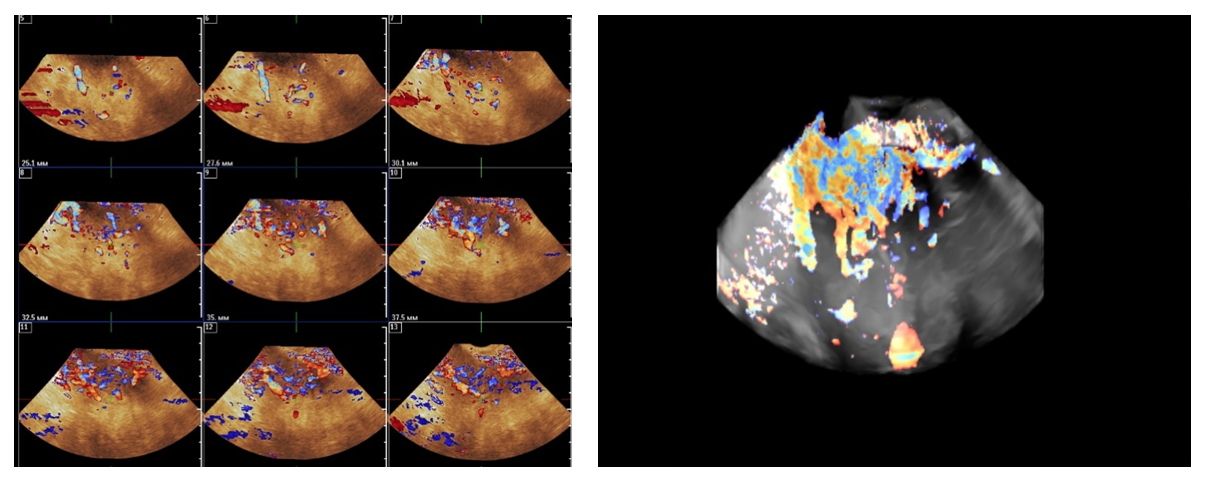
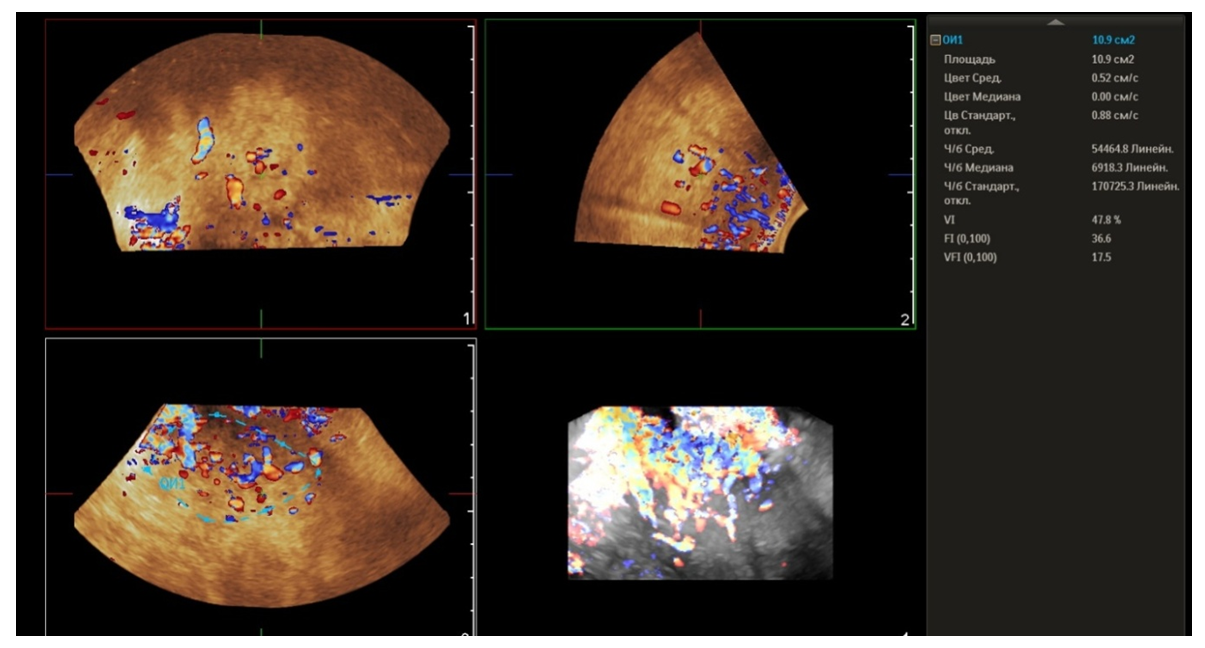
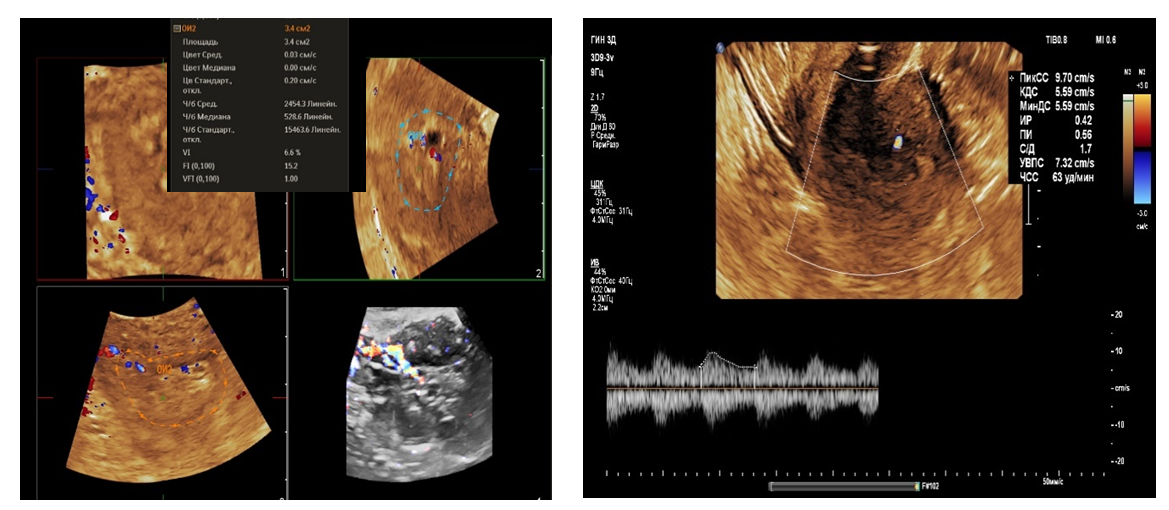
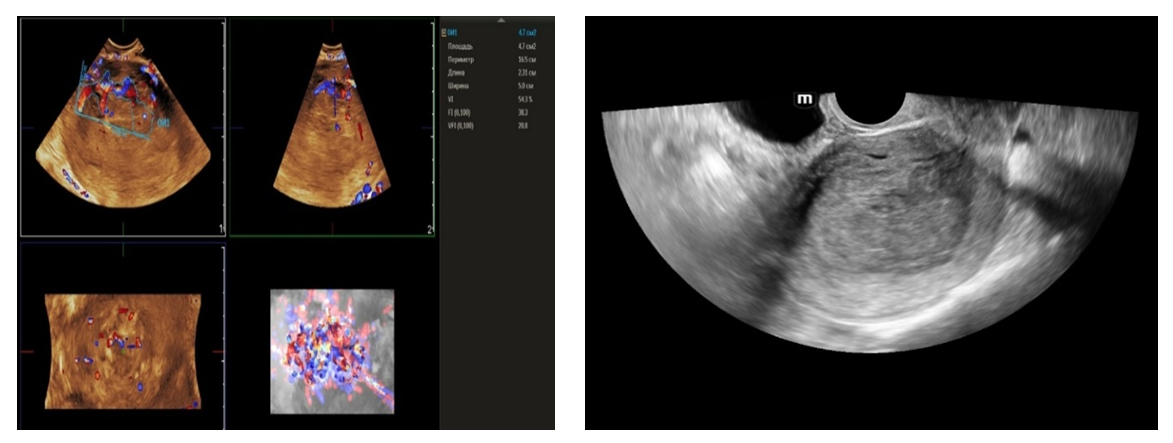
- From April to September 2024, 98 women admitted to the Tashkent regional branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology with complaints of uterine bleeding against the background of menopause lasting 5-15 years were examined. The women's ages ranged from 51 to 72 years (mean age was 61.5 years). Multiparametric ultrasound examination using transabdominal (3.5 MHz detector) and transvaginal (6.5-7.5 MHz detector and 3D detector) accesses was performed using MINDRAY RESONA I9 and PHILLIPS Affiniti 70G devices with the Virtual Organ Computer-aided Analysis (VOCAL) option, which allow a qualitative and quantitative evaluation of the degree of uterine and endometrial perfusion severity. Sixty-five (66.3%) patients from 98 examined women had arterial hypertension; 28 (28.5%) women had diabetes mellitus, 89 (90.8%) women were obese with BMI more than 3.5. Thirty-seven of them (37.7%) had the classic triad of risk factors for endometrial pathology (hypertension, diabetes mellitus and obesity). All women were referred for curettage and diagnostic scraping under general anesthesia. Based on the results of histologic study, the examined patients (n=98) were divided into two groups:Group I consisted of 36 (36.7%) patients with endometrial hyperplasia without atypia. The inclusion criteria were as follows: age of menopause, echographically detected endometrial thickness of more than 5 mm.Group II consisted of 62 (63.2%) women whose histologic examination confirmed endometrial adenocarcinoma of various degrees of differentiation. The selection criteria were the same: age of menopause and thickened endometrium (thickness more than 5 mm).A group of 30 postmenopausal women without atypical bleeding and without gynecologic complaints served as a Control group. The same age selection criterion was used for this group; their mean age was 61.5±10.5 years.The Doppler window was placed over the subendometrial area. The color gain was about 3.4 with normal color quality and PRF 600 (pulse repetition rate), and the wall filter was 50 Hz. The most intense color signal was identified and the sample volume was placed over it to obtain the arterial flow wave. The resistance index (RI) was then automatically calculated and we considered the lowest value.For a more detailed characteristics of the degree of uterine vascularization, in addition to volumetric hemodynamic indices, we calculated the arterial perfusion index (PI). The calculations were performed in accordance with the method described by I.A. Ozerskaya (2013). using the following formula: Arterial perfusion index (API) = (VvolRUA + VvolLUA) / UV,where VvolRUA and VvolLUA — volume blood flow in RUA and LUA, UV — uterine volume (2cm). Volume blood flow in RUA and RUA we determined using the formula: Vvol = V mean × S,where V mean — time-averaged mean blood flow velocity, S — uterine artery area (2cm).The next step was to acquire the endometrial volume in 3D with a 90° viewing angle. The 3D acquisition window was placed above the energy Doppler window. The following measurements were performed in volumetric (3D) angiography mode:- vascularization index (VI) - the number of colored voxels in the total uterine volume; - flow index (FI) - the average value of color in colored voxels, which indicates the "average blood flow intensity" indicator (a number from 0 to 100);- flow vascularization index (VFI) is the average of color in all voxels in a volume, it is a mixed expression of both blood flow and tissue vascularization (number from 0 to 100). All indices were automatically calculated using the VOCAL program. The uterus was scanned in two planes: longitudinally and transversely. Anteroposterior endometrial thickness measurements were obtained from long axis projections. We evaluated the depth of myometrial invasion at all stages of echographic examination by the ratio of the thickness of normal unchanged myometrium to the thickness of the thinned uterine wall in the area of the neoplasm. And we also paid special attention to the spread of the existing malignant process to the cervical canal, which was crucial in determining the stage of the disease according to the International Classification of Endometrial Cancer. Patients with endometrial hyperplasia were treated medically. Patients who were diagnosed as having hyperplasia with cytologic atypia or endometrial cancer were treated surgically. Total abdominal hysterectomy with bilateral salpingo-oophorectomy was performed. Lymph node dissection (para-aortic or pelvic) was also performed in cases with carcinoma. Detailed histopathologic reports were recorded. All tumors were staged according to the International Federation of Gynecology and Obstetrics criteria.
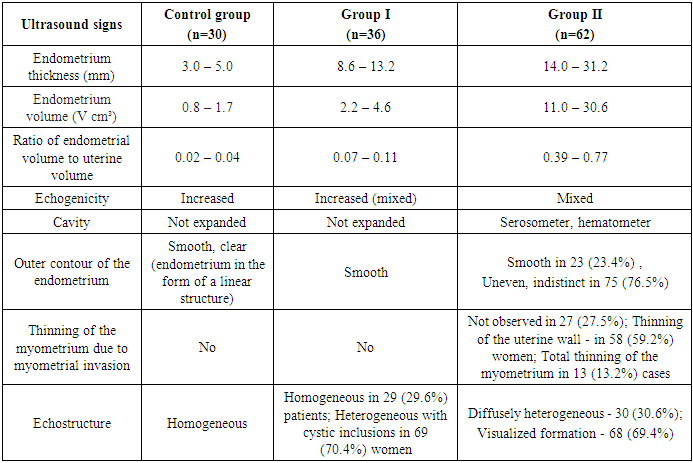
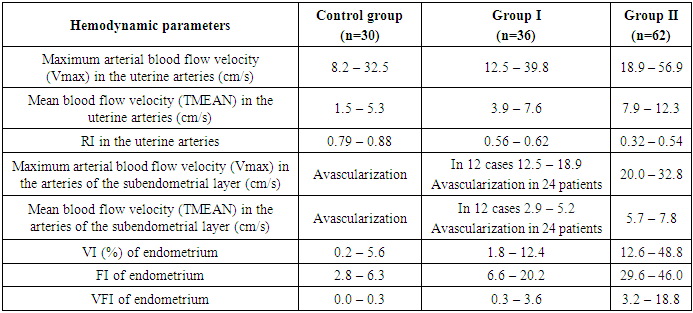
3. Results and Discussion
- In patients of the Control group, who had no complaints of bloody discharge, the endometrial thickness was within 3-5 mm, with a volume of 0.8 - 1.7 cm³ (Tab.1).
|
|
4. Discussion
- The results when both methods were combined were more accurate in differential diagnostics. The resistance index was much lower in patients with low-differentiated carcinoma. Threshold values for different indices were derived to predict endometrial carcinoma. The malignancy thresholds we obtained were VFI - 0.22, VI - 0.7%, FI - 25. VFI was found to be the most accurate index in predicting malignancy compared to other indices. Tumor stage correlated best with VI and FI. Myometrial invasion of more than 50% increases all indices and all foci confirmed as having a high degree of malignancy.Differential diagnostics of benign endometrial hyperplasia and adenocarcinoma is a very important issue in cases of abnormal uterine bleeding in menopausal women, as it is crucial to modify the treatment plan. In addition, predicting the degree of invasiveness and tumor stage before surgery influences the treatment and type of surgery and provides a prognosis for the patient. Complex endometrial hyperplasia and endometrial carcinoma are angiogenic. Moreover, in stage Ib endometrial carcinoma, greater depth of invasion and higher degree of tumor malignancy directly correlate with angiogenic intensity.In cases of postmenopausal bleeding, a thin endometrium less than or equal to 5 mm usually rules out endometrial carcinoma; however, some cases of early carcinoma may have a thin endometrium. The change from endometrial hyperplasia to carcinoma is accompanied by neovascularization and angiogenesis, and these changes can be evaluated by color and energy Doppler studies combined with volumetric studies of uterine hemodynamics. 3D-sonography combined with energy Doppler study of endometrial and uterine arteries proved to be more accurate in differentiating endometrial lesions.The main disadvantage of 2D-ultrasound scanning is the difficulty in obtaining the coronal plane of the uterus because the coronal plane allows visualization not only of the uterine cavity but also of the uterine fundus. Adding 3D-ultrasound scanning to the traditional examination can be beneficial because the coronal plane of the uterus can be easily obtained using the 3D-reconstruction method. 2D-ultrasound scanning also cannot provide information about the endometrial-myometrial junction (EMJ). Changes in the EMJ are considered a key element in the diagnosis of an invasive process. As 3D-ultrasound allows to visualize the coronary plane of the uterus and therefore provides a clear image of the EMJ.
5. Conclusions
- An increase in the degree of hyperplasia and depth of endometrial tumor invasion is accompanied by an increase of angiogenesis, which can also be detected by the uterine arterial perfusion index. It is important in the preoperative evaluation stage. The results of our study indicate that there is a correlation between intratumoral blood flow patterns and histopathological characteristics, tumor stage, and risk of recurrence of endometrial cancer. Further prospective studies are needed to determine the clinical utility of preoperative evaluation of tumor vascularization for these carcinomas.
Conflict of Interests
- The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
ACKNOWLEDGEMENTS
- The authors express their gratitude to the management of the multidisciplinary clinic of Tashkent regional branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology for the material provided for our study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML