-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(11): 2951-2953
doi:10.5923/j.ajmms.20241411.60
Received: Oct. 29, 2024; Accepted: Nov. 21, 2024; Published: Nov. 25, 2024

Indicators of Innate Cell Immunity in Hemolytic Disease of the Fetus in Pregnant Women with Rh-Immunization
G. U. Ashirbekova, U. U. Jabborov, N. Sh. Ergasheva
Republican Perinatal Center of the Ministry of Health of the Republic of Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

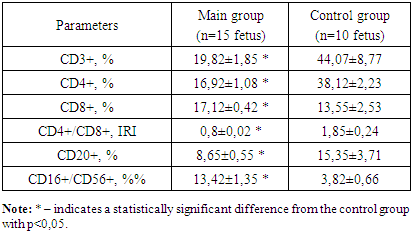
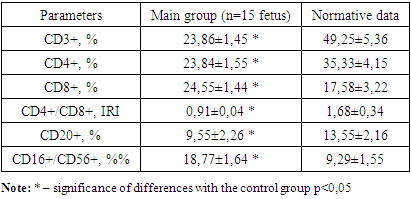
This study assessed immune cell changes in fetuses with hemolytic disease due to Rh immunization. Blood samples from Rh-immunized pregnancies revealed reduced CD3+ T-lymphocytes and CD4+ T-helper cells and elevated CD8+ cytotoxic lymphocytes and CD16+ T-killer cells, indicating active cytotoxic response and potential fetal immunodeficiency. Purpose of the study: the indicators of innate cellular immunity of fetuses with hemolytic disease in pregnant women with Rh immunization. Materials and methods: studies were carried out in theRepublican Perinatal Center for 2024. A total of 40 pregnant women participated in the research and were divided into 3 groups. Group 1: 15 pregnant women with Rh immunization in the second trimester, 2nd group of 15 pregnant women with Rh immunization in the third trimester and 3rd control group of 10 healthy pregnant women. All pregnant women, after their consent, underwent transabdominal cordocentesis to collect blood from the fetal umbilical cord vein. All immunological studies were carried out at the Institute of Immunology of the Academy of Sciences of the Republic of Uzbekistan. Results: the number of CD3+ T-lymphocytes and CD4+ T-helper cells in fetuses with hemolytic disease was significantly reduced in both the II and III trimesters compared to the control group. While the number of CD20+ B-lymphocytes in fetuses is reduced only in the second trimester. The number of CD8+ cytotoxic lymphocytes and CD16+ T-killer cells in fetuses was significantly increased both in the II and III trimesters in relation to the data of the control group. Conclusion: With hemolytic disease both in II and III-trimester, the synthesis of T-lymphocytes by the fetus is significantly reduced, primarily due to the total pool of CD3+, CD4+ T-helper cells, which is the result of the immunological response of the fetus. A significant decrease in CD20+ B-lymphocytes indicates a load on the bone marrow, which is not able to produce a sufficient number of fetal B-lymphocytes. A significant increase in the number of CD8+ cytotoxic lymphocytes and CD16+ T-killer cells shows that the cytotoxic process is active and can be reflected in the formation of profound immunodeficiency in the fetus.
Keywords: Rh-immunization, Hemolytic disease of the fetus, T-lymphocytes CD3+, CD4+, CD8+, CD16+, CD20+ B-lymphocytes, Immunoregulatory index, II, III trimester
Cite this paper: G. U. Ashirbekova, U. U. Jabborov, N. Sh. Ergasheva, Indicators of Innate Cell Immunity in Hemolytic Disease of the Fetus in Pregnant Women with Rh-Immunization, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2951-2953. doi: 10.5923/j.ajmms.20241411.60.
Article Outline
1. Introduction
- Fetal and newborn hemolytic disease (HDFN) is a serious and potentially life-threatening condition caused by the sensitization of the mother’s immune system to fetal red blood cell antigens. Once sensitization occurs, the primary goal of therapy is to minimize complications [1]. The current standard of treatment involves non-invasive monitoring of fetal anemia through repeated ultrasound assessments of the peak systolic pressure in the middle cerebral artery, which indicates anemia when it increases to more than 1.5 times the median (MoM) [2]. If fetal anemia is suspected, invasive procedures like transabdominal cordocentesis and intrauterine transfusions (IUT) may follow [3,4]. In the hands of an experienced specialist, IUT is generally a safe procedure [5]. While generally safe, IUT carries increased risks before 24 weeks or in cases of immune hydrops fetalis. SMFM guidelines recommend the final IUT by 34 weeks and consider labor induction if peak systolic velocity increases after 35 weeks. [6,7,8]. HDFN also rises IgG and IgA levels, increasing immune complexes that may harm fetal tissues. Immunomodulatory treatments, such as plasmapheresis or intravenous immunoglobulin, are currently used in Rh-immunized pregnancies. [9,10,11]. This study aims to analyze key innate cellular immunity indicators in fetuses with HDFN across different gestational ages. The aim of study was to evaluate cellular factors of fetal immunity, including key T-lymphocytes (CD3+), T-helper cells (CD4+), T-suppressor cells (CD8+), T-killer cells (CD16+), and B-lymphocytes (CD20+). Additionally, we sought determine the immunoregulatory index, defined as the ratio of CD4+/CD8+ T-lymphocytes, in the umbilical cord blood of fetuses with hemolytic disease due to Rh incompatibility in pregnant women.
2. Research Materials and Methods
- Studies were conducted at the Republican Perinatal Center in 2024, involving 40 pregnant women divided into three groups. The first group included 15 pregnant women with Rh immunization in the second trimester, the second group comprised 15 pregnant women with Rh immunization in the third trimester, and the third, control group consisted of 10 healthy pregnant women in the second trimester. With informed consent, all participants underwent transabdominal cordocentesis to obtain blood from the fetal umbilical vein. In the second trimester, we used data from reliable sample of 10 fetuses who underwent transabdominal cordocentesis for diagnostic purposes due to suspected congenital development disorders (CDD). As these anomalies were not genetically confirmed, these fetuses were included in the control group. For comparison in the third-trimester, well-documented literature data were referenced as a control. Immunological Research Methods were performed at the Institute of Human Immunology and Genomics, Academy of science, Republic of Uzbekistan. Cellular immunity markers (CD3+, CD4+, CD8+, CD20+, CD16+/56+) were analyzed using BD monoclonal antibodies and flow cytometry (BD Accuri C6). The immunoregulatory index (IRI), defined as the CD4+/CD8+ ratio, was calculated manually, with healthy values typically exceeding one.
3. Statistical Processing of Result
- Statistical analysis was conducted using both parametric and nonparametric methods. Data collection, adjustment, organization, and visualization of result were performed using Microsoft Office Excel 2018. Statistical analysis was carried out with IBM SPSS Statistics v.26 (IBM Corporation). For comparing means in normally distributed quantitative data sets, Student's t-test was applied. The obtained t-test values were evaluated against critical values, with differences in indicators considered statistically significant at a significance level of p < 0.05.
4. Result and Discussion
- We conducted studies on the adaptive (specific) cellular immunity of the fetus. The findings for adaptive cellular immunity in the fetus during the second trimester of pregnancy are presented in table 1.
|
|
5. Conclusions
- 1. In hemolytic disease, regardless of the trimester, there is a decrease in the synthesis of both CD3+ T-lymphocytes and CD4+ T-helper cells in fetuses. This reduction contributes to a decline in CD20+ B-lymphocytes, reflecting a diminished immunological response in the fetus. 2. Elevated levels of cytotoxic CD8+ T-lymphocyte and CD16+ T-killer cells indicate an active cytotoxic process in the fetus during both the second and third trimester, which may lead to the development of severe immunodeficiency. 3. The suppression of the immunoregulatory index (IRI) in fetuses, compared to the control group, is attributed to a decrease in T-helper cells and an increase in T-cytotoxic lymphocytes. A reduced IRI is an important indicator of the severity of T-cell immunodeficiency in fetuses with hemolytic disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML