-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(11): 2919-2924
doi:10.5923/j.ajmms.20241411.52
Received: Nov. 3, 2024; Accepted: Nov. 21, 2024; Published: Nov. 22, 2024

Rh Immunization of Rh Negative Pregnant Women Depending on Phenotype
G. S. Babadjanova, K. A. Sattarova
Tashkent Medical Academy, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

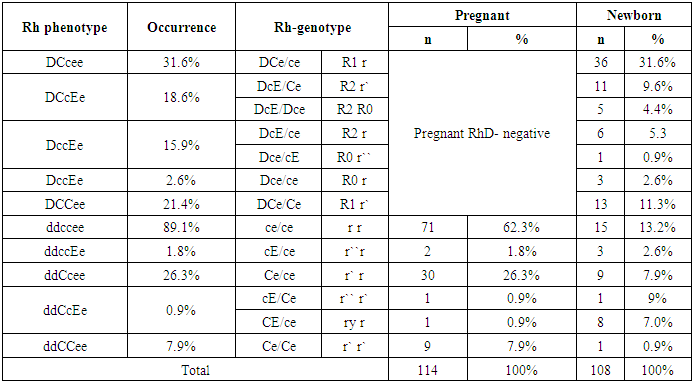
The aim of the study was to study the features of the Rh phenotype in pregnant women with Rh negative blood and to compare the risks of hemolytic disease in the fetus and newborn baby. Background. Currently, early diagnostics of maternal and paternal Rh status, careful anamnesis, use of Dopplerometry, minimization of invasive diagnostic and therapeutic procedures, and avoidance of immunization development in non-sensitized pregnant women are part of the management of pregnant women with Rh immunization. Recently, more than 260 alleles of the RHD gene have been identified in the Rhesus system. Material and methods. 114 pregnant women with Rh negative blood were examined in the Tashkent Interdistrict Perinatal Center #9 for the period from 2021 to 2024. All the examined pregnant women were divided into 3 groups. Blood sampling was done at the first visit of the pregnant woman to the family health clinic, blood sampling of the newborn was done in the delivery room, from the umbilical artery of the placenta. Results. Male sex was slightly predominant among the newborns - 59 (54.1%) ones. The frequency of occurrence of Rh-phenotypes in the studied patients was studied. The results showed that among Rh(D)-positive newborns, the most common phenotype was DCcee - (31.6%), followed in descending order by the phenotypes DCCee - (21.4%), DCcEe - (18.6%) and DccEe - (15.9%). Discussion. Thus, according to the phenotype of the mother with Rh-negative blood type in the presence of a difference from the phenotype of the fetus may depend on the development of hemolytic disease of both the fetus and the newborn. HDFN is developed with the maternal phenotype ccee and the fetal phenotype CCee in 47.9% of cases, with the maternal phenotype ccEe and the fetal phenotype CCee in 50.0% of cases, with the maternal phenotype Ccee and the fetal phenotype CCee in 50.0% of cases, and with the fetal phenotype Ccee in 100% of cases.
Keywords: Rh immunization, Rh group phenotypes, Hemolytic disease of the newborn, Prevention of Rh immunization of the fetus
Cite this paper: G. S. Babadjanova, K. A. Sattarova, Rh Immunization of Rh Negative Pregnant Women Depending on Phenotype, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2919-2924. doi: 10.5923/j.ajmms.20241411.52.
Article Outline
1. Introduction
- The antigens of the rhesus system are encoded by two genes, RHD and RHCE. It was proven in 1996 [1]. The RHD gene determines the synthesis of the protein molecule of the RhD antigen and its allelic variants. Recently, more than 260 alleles of the RHD gene have been identified in the Rhesus system. The molecular bases for the formation of allelic variants and polymorphisms of RHCE and RHD genes are similar. They are caused by single or multiple mutations or segmental substitutions of parts of the RHCE gene for parts of the RHD gene, i.e. the formation of hybrid RHCE-D-CE genes [1]. The algorithm for typing Rhesus system antigens is different for RhD antigen and RhCsee antigens. The detection of RhD antigen is performed in stages: first by erythrocyte agglutination reaction with complete anti-D-antibodies, and if the result is negative, more sensitive techniques using incomplete anti-D-antibodies are performed. The classical D antigen consists of 37 constituent epitopes [2]. Among them are three main ones: weak D antigen - D weak (its amount on the erythrocyte is reduced), partial D antigen that lacks any of the epitopes (individuals with such D antigen can produce antibodies to their missing epitopes) and DEL. The existence of partial RhD antigens was first suggested by A. S. Wiener and L. J. Unger [3], P. Tippett and R. Sanger [4], who described patients with expressed or attenuated D antigens. Some patients were able to produce anti-D antibodies. The researchers concluded that there was a distinct variant of D antigen that had qualitative differences from the well-characterized D antigen. The phylogeny of the human RHD gene proves the existence of four major clusters, which are distinguished by alleles that differ from the normal alleles of the RHD gene and include D antigen variants with additional amino acid substitutions: DIV, DAU, weak D weak type 4 and Eurasian. The D weak type 4, DIVa and DAU clusters are associated with the cDe haplotype and are found predominantly in black people, while the cde and cDE haplotypes are associated with the Eurasian cluster and are represented in white people. The Eurasian D-cluster includes, in particular, the partial antigens RhDNB and RhDVII. These partial antigens are both associated with the CDe haplotype and arise from single point mutations. The RhDNB antigen is referred to as RHD*25 or RHD*DNB by ISBT nomenclature. This gene results from a nucleotide substitution of glutamine for serine at position 355 and is characterized by the absence of epitopes 6, 31. According to its serologic properties, the RhDNB antigen is classified as a weakly expressed antigen. The RhDVII antigen was discovered in 1995. This antigen is formed by a nucleotide substitution of leucine with proline at position 110 and differs from the normal RhD antigen by the absence of epitope 8 [3]. The modern strategy for the management of pregnant women with Rh immunization includes early diagnostics of maternal, paternal, and fetal Rh status using maternal blood, accurate assessment of the severity of fetal anemia, use of a minimum number of invasive diagnostic and therapeutic procedures, and avoidance of immunization in non-sensitized pregnant women. The critical importance of preventive measures should be emphasized. Studying the pathogenesis, diagnosis, treatment, and prevention of fetal hemolytic disease, the following potentially etiopathogenetic events are highlighted: invasive prenatal diagnostics, reduction of one of the embryos, intrauterine treatment of the fetus (shunting, transfusions), abdominal trauma, intrauterine fetal death, termination of pregnancy (regardless of the method), antepartum hemorrhage, spontaneous abortion, ectopic pregnancy. Throughout the gestational period, immunobiologic interactions occur between the maternal body and the fetus, which largely determine the subsequent course of pregnancy and the outcome for the newborn. Immunoconflicted pregnancy occupies a special place in the composition of obstetric complications. About 95% of all clinically recognized cases of fetal/newborn hemolytic disease (HDFN) are due to Rh incompatibility [5]. According to literature data, HDFN is the 2nd most common cause of stillbirth and is diagnosed in 0.5% of newborns [5]. In the structure of perinatal mortality hemolytic disease of newborns is on the 4-6th place. Despite significant advances in the field of perinatology, many issues of management of pregnant women with Rh-immunization, diagnostics and treatment of hemolytic disease of the fetus and newborn cannot be considered resolved. Among the immunological complications of pregnancy, the first place is occupied by hemolytic disease of the fetus and newborn, which develops as a result of incompatibility of maternal and fetal blood on various erythrocyte antigens. Perinatal mortality rates in fetal hemolytic disease are 15-16‰ [4]. Significant reduction of perinatal morbidity and mortality from fetal hemolytic disease is impossible without organization of timely and universal prevention of Rhesus immunization during pregnancy and early postpartum period at the population level [7]. For specific prevention, human anti-Rhesus immunoglobulin Rho (D) is used - resonative or rogam, which allows to minimize the risk of Rhesus sensitization in pregnant women, significantly reducing the level of mortality and disability from hemolytic disease of the fetus and newborn. It is known that there is a correlation between HDN severity and antibody specificity. The most severe course of HDN is caused by the presence of antibodies to the Rhesus system antigen "D" and "c" in the mother; antibodies of anti-E and anti-C specificity can cause moderate HDN, and anti-Lea, anti-Kpa and anti-Lua do not cause HDN. According to the literature, post-transfusion complications (PTC) and reactions are more common in women with a history of pregnancy [9]. Antibodies of specificity anti-D, anti-c, anti-K, anti-K, anti-k, anti-Jka are considered the most dangerous ones [10]. It was found that over time in alloimmunized patients, antibodies stop being produced and their concentration becomes insufficient to be detected in vitro even by the most sensitive methods of investigation. Repeated transfusion of donor red blood cells containing an antigen to which the recipient has antibodies stimulates the production of antibodies and increases their concentration in the recipient's blood. The problem of isoserologic incompatibility between mother and fetus is of practical interest to obstetrician-gynecologists, neonatologists and transfusiologists [3-5]. The inheritance of Rh-antigens is determined by a series of allelic genes located closely on one chromosome, with genes D and d, C and c, E and e being in mutually exclusive relationships, i.e. if the D gene is present on the chromosome, the d gene is absent and vice versa. All three genes on one chromosome are inherited simultaneously. The inheritance of Rh antigens does not occur through individual antigens D, C, e, but through a whole complex of antigens combined together “D C e”. Considering the 6 major alleles of Rh antigens, there are 8 major combinations of Rh antigens. However, different combinations of Rh-antigens do not occur with equal frequency. If we take into account that a child inherits one gene from each parent, then there are at least 36 possible genotypes of the Rh-system [1]. According to the world's leading centers, sensitization of women of reproductive age to antigens of the Rhesus system occurs in: anti-D - 25%, anti-C - 7%, anti-E - 18%, anti-C - 6% [2,5].These antibodies are more often formed in Rh-positive individuals as a result of hemotransfusion and/ or repeated pregnancies.The aim of the study was to study the features of the Rh phenotype in pregnant women with Rh negative blood and to compare the risks of hemolytic disease in the fetus and newborn baby.
2. Material and Methods
- In order to achieve the set goals and objectives, 114 pregnant women with Rh negative blood were examined in the Tashkent Interdistrict Perinatal Center #9 and the Scientific Production Enterprise "Blood Preparations" for the period from 2021 to 2024. All the examined pregnant women were divided into 3 groups: • Group 1A (n=51) - women who were pregnant again with Rh-negative blood without immunization and who gave birth to Rh-positive newborns with signs of hemolytic disease of the fetus/newborn (HDFN); • 1B group (n=29) consisted of repeat pregnant women with Rh-negative blood without immunization who gave birth to Rh positive newborns without HDFN signs; • Group 1С (n=34) are women who are pregnant again with Rh-negative blood type without immunization and who gave birth to Rh-negative newborns. All patients included in the study underwent a complete clinical and laboratory examination, as stipulated in the national clinical protocol of health care of the Republic of Uzbekistan for the management of normal pregnancy, as well as diagnostics and treatment of isoserologic incompatibility of maternal and fetal blood. Immunohematologic examination of pregnant women, including screening of anti-erythrocyte antibodies in indirect antiglobulin test, antibody phenotyping, phenotyping of pregnancy antigens according to the Rhesus and Kell systems, and determination of IgG immunoglobulin subclasses were performed using a laboratory centrifuge, ID-cards, and standard red blood cells of BIO RAD (France/USA).The newborn's condition characteristics included Apgar score at the 1st and 5th minute, determination of weight and height parameters, comprehensive clinical and laboratory examination with determination of hemoglobin level, hematocrit, and total bilirubin and its fractions. The study of phenotype by Rh-antigens, blood group of the newborn from umbilical cord blood taken at the time of birth were carried out. Blood sampling was done at the first visit of the pregnant woman to the family health clinic, blood sampling of the newborn was done in the delivery room, from the umbilical artery of the placenta. The women were being interviewed at the time of testing and written consent was obtained. Gel technology in microtubes (GTM) was proposed by Y. Lapierre in 1989. The method is based on agglutination of erythrocytes in agar gel “Sephadex” placed in microtubes. The system is usually plastic cards containing gel-filled microtubes (DiaMed AG patent, Switzerland). The blood under study was added to the top of the microtubes of the diagnostic cards and centrifugation was performed to separate agglutinated and non-agglutinated erythrocytes as follows: non-agglutinated erythrocytes have a size comparable to the size of gel particles and freely pass through them under the action of centrifugal force, forming a compact red precipitate at the bottom of the microtube - negative result; agglutinated erythrocytes due to their large size are retained on the gel surface or in its thickness - positive result. Fixed agglutination in the gel allows easy evaluation of the reaction result, and the presence of a control microtube in the diagnostic card confirms the reliability of the obtained data.Depending on the strength of the agglutination reaction in gel medium, the following evaluation of the obtained results is accepted:- strongly positive (++++) - formed agglutinates of erythrocytes are retained on the gel surface;- positive (+++) - agglutinates are located in the upper third of the gel column;- weakly positive (++) - agglutinates are fixed in the upper two thirds of the gel;- very weakly positive (+) - agglutinates are located in the lower third of the gel;- negative (-) - erythrocytes form a compact precipitate at the bottom of the microtube.DiaMed identification cards (ID-cards) are plastic cards that have six microtubes embedded in each card. Five test tubes contain a mixture of gel and antiserum. Each card has a sixth control tube containing neutral gel without antibodies. The labeling of the tubes in the ID card was done by the antigens detected, e.g. A-B-AB-D-CDE-Ctl or C-c-E-e-K-Ctl.The study materials were subjected to statistical processing using parametric and nonparametric analysis methods. Accumulation, correction, systematization of initial information and visualization of the obtained results were carried out in Microsoft Office Excel 2016 spreadsheets. Statistical analysis was performed using IBM SPSS Statistics v.26 program (IBM Corporation).In the selection of Rh-negative pregnant women, blood group affiliation was also taken into account. Thus, pregnant women with the first I(0) blood type did not participate in this study to exclude immunization according to the ABO system.
3. Results
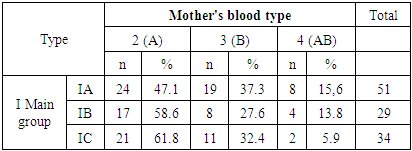
- The mean age was 27.5±0.35 years in group 1A. In group 1B this index was 29.3±1.96 years and in group 1C it was 20.57±0.96 years. Blood group determination according to the AVO system was performed at the initial immunohematologic examination of the patients. At the same time, it was statistically significant that blood group 2 was significantly more frequent. Taking into account that all women were Rh-negative, statistical deduction by Rh identity was not performed (Table 1).
|
|
|
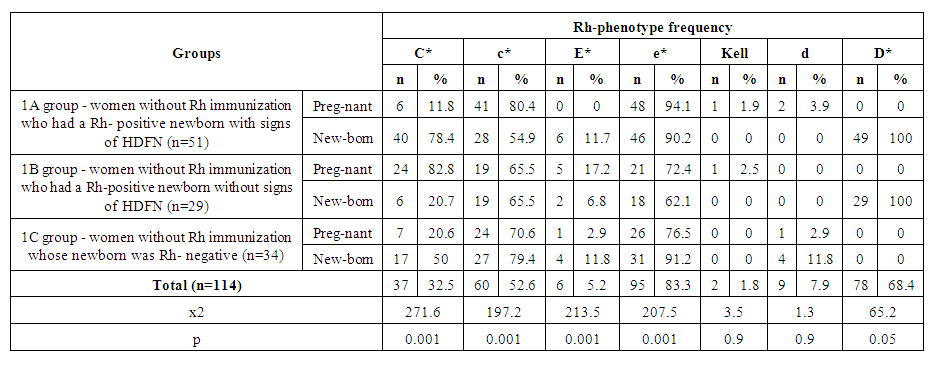 | Table 4. Comparison of Rh-antigen phenotyping of pregnant women and newborns |
4. Discussion
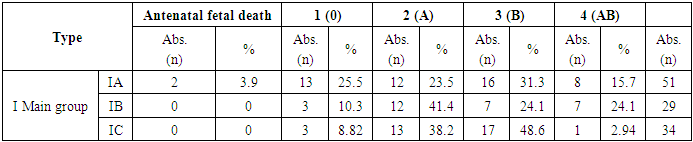
- The data of the analysis showed that in the group of Rh negative pregnant women without immunization (1A) who gave birth to newborns and postnatal hemolytic anemia was developed, the presence of dominant “C” antigen was found in 11.8%, minor “c” - in 80.4% of cases, as well as minor “e” Rh antigen was detected in 94.1% of cases, while dominant “E” antigen was not observed in this group. Phenotyping of the blood of newborns of this group revealed the presence of dominant “C” antigen in 78.4% of cases, which substantiates the development of hemolytic disease of newborns by “C” antigen. In statistical analysis, the data were prognostically significant (р<0.05) [11]. In newborns with Rhesus negative blood, in 4 cases (11.7%) babies with signs of hemolytic disease were observed. When phenotyping was performed, there were differences between the Rh-antigens of the mother and the newborn. In this case, the phenotype of mothers had a “Csee” pattern, while the phenotype of newborns was “Csee, Csee” [12]. A correlative evaluation of the development of hemolytic disease in newborns independent of RhD-antigen was performed. Thus, according to the phenotype of the mother with Rh-negative blood type in the presence of a difference from the phenotype of the fetus may depend on the development of hemolytic disease of both the fetus and the newborn. HDFN is developed with the maternal phenotype "ccee" and the fetal phenotype "CCee" in 47.9% of cases, with the maternal phenotype "ccEe" and the fetal phenotype "CCee" in 50.0% of cases, with the maternal phenotype "Ccee" and the fetal phenotype "CCee" in 50.0% of cases, and with the fetal phenotype "Ccee" in 100% of cases.
5. Conclusions
- Thus, in the prognosis and prevention of fetal hemolytic disease, blood group and Rh-factor analysis alone is not always informative. Phenotyping of maternal blood for Rh antigens is a significant prognostic factor, as detection of recessive c (78.4%) and e (90.2%) antigens in maternal blood increases the risk of postnatal neonatal hemolytic disease in women without immunization in the presence of major C and E antigens in fetal blood (OR=2.97; RR=1.39). This index significantly increased in the presence of such factors as repeated pregnancy (OR=6.02; RR=1.51), reproductive losses in history (OR=4.1; RR=2.09), presence of increased blood flow in the middle cerebral artery (OR=4.43; RR=2.40).
Conflict of Interests
- The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
ACKNOWLEDGEMENTS
- The authors express their gratitude to the management of the multidisciplinary clinic of Tashkent Interdistrict Perinatal Center #9 for the material provided for our study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML