-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(11): 2909-2911
doi:10.5923/j.ajmms.20241411.50
Received: Oct. 29, 2024; Accepted: Nov. 17, 2024; Published: Nov. 21, 2024

Significance of Endothelial Markers in Thrombocytopenia and Thrombocytopathy in Pregnancy
Zaynutdinova Dilafruz, Babadjanova Shaira, Nuriddinova Nodira, Bekchanova Nazokat
Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Endothelial dysfunction plays a crucial role in the pathogenesis of thrombocytopenia and thrombocytopathy, particularly during pregnancy. Elevated endothelial markers such as Endothelin-1, sICAM-1, and Willebrand factor exacerbate these conditions by promoting inflammation, which increases the risk of severe hemorrhagic complications, endangering both maternal and fetal health. This study examines the presence and levels of endothelial markers in pregnant women with thrombocytopenia and thrombocytopathy, aiming to elucidate their role in disease progression and potential as therapeutic targets. The findings underscore the need for tailored management strategies to mitigate the associated risks in pregnancy.
Keywords: Thrombocytopenia, Thrombocytopathy, Pregnancy, Endothelin-1, sICAM-1, Willebrand factor
Cite this paper: Zaynutdinova Dilafruz, Babadjanova Shaira, Nuriddinova Nodira, Bekchanova Nazokat, Significance of Endothelial Markers in Thrombocytopenia and Thrombocytopathy in Pregnancy, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2909-2911. doi: 10.5923/j.ajmms.20241411.50.
1. Introduction
- Hemostasis stimulates platelet function, directly contributing to the growth of endothelial markers. These include Willebrand factor, P-selectin, endothelin-1 and ICAM-1 (intracellular adhesion molecule-1 or CD54) [5]. Willebrand platelet factor, megakaryocytic, plasma and endothelial receptors contain a multimeric glycoprotein that destroys the endothelium, which is a subendothelial platelet factor and an inhibitory factor of the Willebrand receptor and GPIb receptor in platelets. From this, Willebrand the factor of erection regeneration and platelet aggregation of the oral canal strives to ensure that integrin aIIbb3 stimulates and stimulates aggregation [2,12].Whether the number of platelets is insufficient despite the Willebrand factor being sufficient or the failure of the GPIb receptor on the cell surface causes this bridge to not form. This leads to life-threatening bleeding during labor [4]. Endothelial cells line the blood vessels, lymph vessels and heart chambers and form a boundary between the vessel on one side and the blood or lymph fluid on one side [9]. Such a strategic position of the endothelium determines its most important functional role in regulating vascular tone, hemostasis and inflammatory processes [10]. Damaged endothelium can be the cause and result of many diseases [3,8].In all types of inflammation, the endothelial barrier integrity is disrupted, which leads to damage to endothelial cells. As a result, the activity of hemostasis is disrupted by increased production of cytokines and reactive oxygen. Plasma endothelin-1 also increases with the severity of the disease in immune thrombocytopenia [7].The intercellular adhesion molecule ICAM-1 and VCAM-1 (Vascular cell adhesion molecule 1 or CD106) are important for the pathogenesis of inflammations in the body. ICAM-1 (intercellular adhesion molecule-1) is a transmembrane protein expressed in endothelial and epithelial cells at inflammatory sites. It is known that the levels of intercellular adhesion molecules, in particular the levels of sICAM-1, sVCAM-1 and cytokines (e.g. IL-6, TNF-a), indicate reliable signs of inflammation and the effectiveness of pathogenetic therapy [6].There are reports in the literature that anti-inflammatory cytokines activate endothelial cells, intercellular adhesion molecules (VCAM-1 and ICAM-1), and selectins activate expression induction [11]. Damaged endothelial cells secrete large amounts of biologically active substances ICAM-1 (CD54) and VCAM-1 (CD106), which are practically not synthesized under physiological conditions. The primary function of ICAM-1 (CD54) is to ensure that neutrophils, monocytes and lymphocytes adhere to the activated vascular endothelium, and then extravasate them and move them to the site of inflammation. For this reason, sICAM-1 in the blood increases in various autoimmune and inflammatory diseases [1].
2. Materials and Methods
- Platelet pathologies were in the form of thrombocytopenia, thrombocytopathies, and 55% (n=60) of the 108 patients studied in total in a prospective study were thrombocytopenia, and 45% (n=48) were thrombocytopathy. The diagnosis was made based on patient complaints, life and medical history, clinical signs and laboratory data. Special attention was paid to the medical history of the disease, objective data and laboratory indicators.All pregnant women who participated in the study were divided into three groups: 1. Main (first) group (n=60) – pregnant women with thrombocytopenia; 2. Main (second) group (n=48) – pregnant women with thrombocytopathy; 3. Control (third) group (n=30) physiological pregnant women with normal platelet count and function. The study involved all patients at will. Immunoenzyme studies in Mindray BA96A (China) spectrophotometer, Shanghai Coon Koon Biotech Co. Ltd (China) was conducted using reagents. The Immunoenzyme examination method aims to identify anti-platelet summar antibodies as well as endothelial dysfunction markers. Method principle: nonconcurrent is a 2-step immunoenzyme analysis, in Step 1, platelet antibodies in the sample are combined with immobilized antigens at the bottom of the tablet lunges. In Step 2, the color change of the combined antibodies is caused by the conjugate celebrated with peroxidase. Sequence of execution of the analysis. The desired strip and lunges were separated from the tablet, and 30 minutes were left in the room layout. A washer solution was prepared that would be needed: distilled water was added to the washer liquid in the volume, which came in the instructions. A working conjugate was prepared: a solution liquefying the conjugate was added to the conjugate concentrate in volume which was brought in the instructions. A working substrate mixture was prepared: a substrate buffer was added to tetramethylbenzidine in the volume, which was brought in the instructions.Standards, control solutions and samples were laid in the volume, which were brought to the microplate in the instructions. With extirpation, the lunges were shaken, the top was closed with a tape and incubated at 370s for 60 Minutes in a thermostat. After incubation, the tape was taken and washed 4-5 times with a washing solution. The filter was gutted with strip and lunges on paper.A 50-100 MCL stop-reagent was inserted into the microplate at the volume, which was brought to the instructions, and an optical density was measured within 15 minutes at 450 nm using a 2-Wave Spectrometer, and a reference-filter at 540-550 nm.The composition of the Villebrand factor was determined using the Immunoenzyme method in blood plasma to determine the values. Technoclone reagents (China) were used. Venous blood was collected in plastic tubes using 3.8% (0.11 mmol/l) sodium citrate (9:1) as an anticoagulant and then centrifuged for 15 minutes at a rate of at least 2,500 g. To determine the level of the Willebrand factor, the analysis procedure is included several stages: incubation with samples and conjugate, working solution to the inlet wells (50 RS), final incubation temperature 37 – 45 min. The calculation of the results was carried out on the calibration curve – x-axis: the concentration of the Willebrand factor on the unit Ed/ml, Y-axis: absorption.
3. Results and Discussion
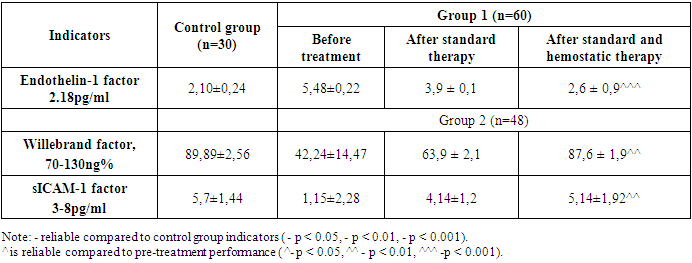
- In patients with thrombocytopenia examined, progression of the disease is observed as a result of endothelial dysfunction disorders, thus increasing bleeding. Endothelial dysfunction has been investigated as laboratory markers for endothelin 1 (ET-1), adhesion molecule (sICAM-1), Willebrand factor (vWF). Changes in these indicators are responsible for a violation of the balance in the blood clotting process, damage to the endothelium of blood vessels, the release of various rashes on the skin, the 100 MCL of working conjugate solution is applied to the microplate, the top is closed with a plinth and incubated in the thermostat for 30 minutes at 370s. After incubation, the tape was taken and washed 4-5 times with a washing solution. The filter was gutted with strip and lunges on paper. 100 MCL of working substrate mixture was applied to the microplate, the top was closed with a plinth, and 20 minutes in the thermostat were incubated in a black field at 18-250s. progression of the disease [91; B. 2269-2273]. The results of testing for endothelial dysfunction in patients with immune thrombocytopenia are shown in Table 1.
|
4. Conclusions
- This study highlights the significant role of endothelial dysfunction markers—Endothelin-1, sICAM-1, and Willebrand factor—in the progression of thrombocytopenia and thrombocytopathy during pregnancy. Elevated levels of these markers correlate with increased risks of hemorrhagic complications, which pose serious threats to maternal and fetal health. The findings underscore the importance of early detection and targeted management strategies focused on endothelial health. Interventions that stabilize endothelial function may reduce complications and improve pregnancy outcomes for women affected by these platelet disorders. Further research into tailored therapies that address endothelial dysfunction in such cases is recommended to enhance both maternal and neonatal safety.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML