-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(11): 2905-2908
doi:10.5923/j.ajmms.20241411.49
Received: Oct. 28, 2024; Accepted: Nov. 16, 2024; Published: Nov. 21, 2024

Significance of Endothelial Dysfunction in the Development of HCV Etiology Chronic Hepatitis and Liver Cirrhosis
N. F. Nuriddinova, Z. Ch. Kurbonova, D. L. Zaynutdinova, N. I. Bekchanova
Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The disruption of intragepatic hemodynamics is a key factor in the pathogenesis and progression of liver diseases, particularly liver cirrhosis and chronic hepatitis. This hemodynamic alteration leads to endothelial dysfunction, a condition that triggers the release of biologically active substances impacting the balance between pro-coagulant and anticoagulant factors. Such an imbalance contributes to a pro-thrombotic state in the liver, which exacerbates intrahepatic fibrosis, cellular apoptosis, and other degenerative changes, accelerating disease progression. This abstract explores the significance of endothelial dysfunction as both a cause and consequence in chronic liver disease, highlighting its role as a potential target for therapeutic intervention aimed at preventing or slowing the advance of cirrhosis. Addressing endothelial damage may thus offer new avenues for treatment strategies, improving outcomes for patients with chronic liver disease.
Keywords: Liver cirrhosis, Hepatitis, Endothelial dysfunction, Endothelin-1, sICAM-1, Willebrand factor
Cite this paper: N. F. Nuriddinova, Z. Ch. Kurbonova, D. L. Zaynutdinova, N. I. Bekchanova, Significance of Endothelial Dysfunction in the Development of HCV Etiology Chronic Hepatitis and Liver Cirrhosis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2905-2908. doi: 10.5923/j.ajmms.20241411.49.
1. Introduction
- Endothelial cells of liver sinusoids not only act as a barrier between the sinusoids and the liver parenchyma, but are also actively involved in the inflammatory reaction, enhancing adgesia and antigen production, eliminating anti-inflammatory agents and producing inflammatory mediators [11].At the same time, the effect of endothelium on aggregation is related to the production of prostacyclin and nitric oxide. The mechanism of action of prostacyclin and nitric oxide is the release of calcium ions from platelets, which reduces the aggregation function of platelets. The anticoagulant effect of the endothelium is related to the production of endogenous heparins, tissue thromboplastin inhibitor, tissue plasminogen activator, thrombomodulin, antithrombin III. Endothelial dysfunction predicts the pathogenesis of a number of diseases. Nowadays, the pathogenetic role of endothelial dysfunction is recognized and it is an important link in the development of cardiovascular diseases, diabetes, bronchial asthma, oncological diseases, intoxication, infections. The function of the vascular endothelium is important in treatment and prevention practices, which leads to the emergence of a new strategic concept of vascular medicine [9].Endothelial cells are damaged under the influence of immune complexes, inflammatory mediators, and viruses. Coagulation factors of the hemostasis system are produced by endothelial cells and hepatocytes, which ensure the interdependence of liver function and vascular endothelium. Endothelial dysfunction, which is the cause of many pathological processes, develops in liver diseases. Endothelial damage leads to the production of biologically active substances, which disrupts the balance between the synthesis of coagulation and anticoagulant factors. In endothelial dysfunction, the balance between production of vasodilation, angioprotective, prothrombotic and proliferative factors is disturbed [5].Violation of the functional state of the endothelium leads to an increase in the cytolytic process in the liver. Won Willebrand factor, which is an indicator of the hemostasis system, increases in liver cirrhosis. Hollestelle M.J. study showed that the amount of Won Willebrand factor in the plasma increases by 10 times during the decompensation stage of liver cirrhosis. This number of platelets helps to compensate for their reduced adhesion function [3].In patients with liver cirrhosis, binding of platelets to Willebrand factor is reduced by 50%, which increases the risk of hemorrhagic complications. [2]. Regardless of the etiology, the activity of Willebrand factor is much higher in chronic diffuse liver diseases [7].Many factors of blood coagulation are synthesized by endothelial cells and hepatocytes, so the liver and endothelium have an effect on hemostasis. In chronic diffuse liver diseases, the spectrum of biologically active substances produced by the endothelium changes, which leads to an imbalance between the synthesis of prothrombogenic and antithrombotic, dilatant and spastic factors. As a result, the synthesis of vasoconstrictors and procoagulants increases, which leads to vessel spasm. As a result of long-term exposure to a harmful factor, the endothelium begins to cause a number of systemic pathological processes (inflammation, thrombosis, etc.) [1]. Death and fibrosis of the liver parenchyma in patients with cirrhosis of the liver combined with repeated thrombosis or hypercoagulation thrombotic complications (in the portal vein system, mesenteric veins, liver veins, veins of limbs, pulmonary embolism); causes the development of porto-pulmonary syndrome (lung endothelial dysfunction, microvascular thrombosis in the lungs). Thrombosis of the portal vein occurs in 0.6–26% of patients with liver cirrhosis. The prevalence of portal vein thrombosis increases with advancing liver disease [4]. Hypercoagulation associated with chronic liver disease can lead to liver parenchymal damage and death. In the last ten years, laboratory diagnostics of hemostasis and fibrinolysis system in chronic diffuse liver diseases have undergone significant changes. Standard complex tests for detection of hemostasis diseases lose their diagnostic value in this group of patients [10]. The problem of predicting hemorrhagic syndrome in liver cirrhosis with the help of modern laboratory tests remains open. There is no standard strategy for the treatment and prevention of hemorrhages in chronic diffuse liver disease. Randomized controlled trials are needed to evaluate laboratory tests in predicting bleeding or thrombosis in patients with cirrhosis.
2. Materials and Methods
- Data were collected from patients treated with chronic hepatitis and cirrhosis of the liver in the departments of hematology and hepatobiliary pathology of the 1st clinic of the Tashkent Medical Academy in 2020-2023. Special attention was paid to the medical history of the disease, objective data and laboratory indicators.Patients in the main group were divided into the following groups:1. Main (first) group (n=20) – chronic hepatitis of HCV etiology without receiving antiviral therapy; 2. Main (second) group (n=30) – chronic hepatitis of HCV etiology receiving antiviral therapy of sofosbuvir and velpatasvir; 3. Main (third) group (n=20) - liver cirrhosis of HCV etiology without receiving antiviral therapy;4. Main (forth) group (n=30) - antiviral therapy of Sofosbuvir and Velpatasvir patients with liver cirrhosis receiving therapy.Immunoenzyme studies in Mindray BA96A (China) spectrophotometer, Shanghai Coon Koon Biotech Co. Ltd (China) was conducted using reagents. The Immunoenzyme examination method aims to identify anti-platelet summar antibodies as well as endothelial dysfunction markers. Method principle: nonconcurrent is a 2-step immunoenzyme analysis, in Step 1, platelet antibodies in the sample are combined with immobilized antigens at the bottom of the tablet lunges. In Step 2, the color change of the combined antibodies is caused by the conjugate celebrated with peroxidase. Sequence of execution of the analysis. The desired strip and lunges were separated from the tablet, and 30 minutes were left in the room layout. A washer solution was prepared that would be needed: distilled water was added to the washer liquid in the volume, which came in the instructions. A working conjugate was prepared: a solution liquefying the conjugate was added to the conjugate concentrate in volume which was brought in the instructions. A working substrate mixture was prepared: a substrate buffer was added to tetramethylbenzidine in the volume, which was brought in the instructions.Standards, control solutions and samples were laid in the volume, which were brought to the microplate in the instructions. With extirpation, the lunges were shaken, the top was closed with a tape and incubated at 370s for 60 Minutes in a thermostat. After incubation, the tape was taken and washed 4-5 times with a washing solution. The filter was gutted with strip and lunges on paper.A 50-100 MCL stop-reagent was inserted into the microplate at the volume, which was brought to the instructions, and an optical density was measured within 15 minutes at 450 nm using a 2-Wave Spectrometer, and a reference-filter at 540-550 nm.
3. Results and Discussion
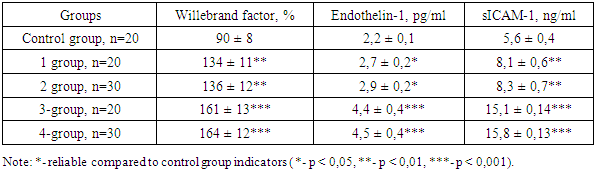
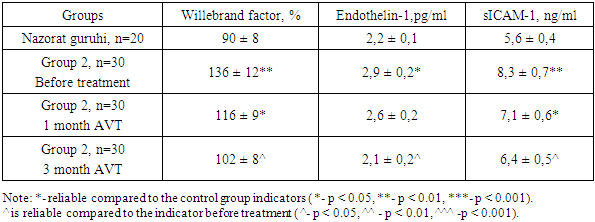
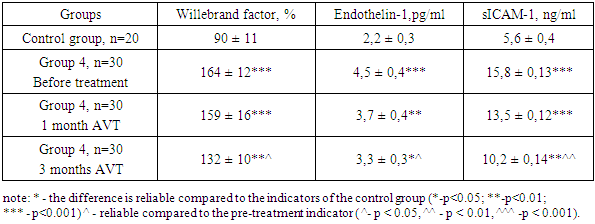
- In chronic diffuse liver diseases of viral etiology, the progression of the disease is observed due to endothelial dysfunction and hemodynamic disturbances in the liver, and because of this, chronic hepatitis turns into liver cirrhosis. Endothelin-1 (ET-1), adhesion molecule (sICAM-1), Willebrand factor (vWf) were examined as laboratory markers of endothelial dysfunction. A change in these indicators causes an imbalance in the blood coagulation process, damage to the endothelium of blood vessels, various rashes on the skin, and progression of the disease. [6]. The results of examination of endothelial dysfunction in chronic diffuse liver diseases with HCV etiology are presented in Table 1.
|
|
|
4. Conclusions
- This study highlights the significant role of endothelial dysfunction in the progression of chronic liver diseases, particularly those of HCV etiology. Increased levels of endothelial dysfunction markers such as Willebrand factor, Endothelin-1, and sICAM-1 underscore the heightened risk of thrombotic and inflammatory responses, especially in advanced liver cirrhosis. These markers reflect a disrupted balance between pro-coagulant and anticoagulant factors, leading to vascular complications that exacerbate liver damage. The findings demonstrate that antiviral therapy (AVT) positively influences endothelial function, reducing the levels of these markers and suggesting an improved vascular environment. Given these outcomes, endothelial dysfunction should be regarded as a key therapeutic target for managing and potentially mitigating the progression of chronic liver disease. Future studies should continue exploring targeted therapies that address endothelial health to enhance the prognosis for patients with HCV-related liver diseases.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML