Ju Seunghwan1, Shamansurova Z. M.2, 3, Salakhov T. A.4, Ismailov S. I.1
1Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
2Central Asian University, School of Medicine, Tashkent, Uzbekistan
3Institute Biophysics and Biochemistry at the National University of Uzbekistan
4Republican Centre of Nephrology and Kidney Transplantation, Tashkent, Uzbekistan
Correspondence to: Shamansurova Z. M., Central Asian University, School of Medicine, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Objective: People on Programmed Hemodialisys due to abnormal minerals level have higher risk of secondary hyperparathyroidism. The purpose of this work was determine of increasing and compare the level of parathyroid hormone in blood plasma, also calculate the risk of SHPT in people on programmed hemodialysis. Material and methods: Data from medical records of 465 patients on programmed hemodialysis who were admitted between 2016-2020 to the Republican Centre of Nephrology and Kidney Transplantation and Republican Specialised Scientific Practical Medical Centre of Endocrinology were analyzed. Patients according to reason of end stage of renal failure were divided into 3 groups due to chronic glomerulonephritis (CGN), diabetes mellitus (DM) and other reasons. Results: The level of glycemia was significantly higher in the group of patients with DM, the level of creatinine, urea nitrogen, calcium, phosphorus in the blood plasma were higher reference range and no differences were found between the groups. In 15% of patients on Programmed hemodialysis PTH level in blood plasma were measured, while 52% among them have a normal range and 48% have a higher levels of hormone. Comparison shown more severe cases of increasing and relative risk were more higher in patients with DM. Conclusion: blood PTH level were measured only in those who were suspected to SHPT and consist 15% of patients on programmed hemodialysis and suggested about low awareness. Among these 15% patients increased blood PTH level were found almost in half (48%). That results suggested about high risk of SHPT among patients on programmed hemodialysis, especially with DM and need to be frequently screened to SHPT by measure blood serum PTH level.
Keywords:
Diabetes mellitus, Chronic glomerulonephritis, End stage of renal disease, Hemodialysis, Hyperparathyroidism, Parathyroid hormone
Cite this paper: Ju Seunghwan, Shamansurova Z. M., Salakhov T. A., Ismailov S. I., Risk of Secondary Hyperparathyroidism in People on Programmed Hemodialysis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2895-2899. doi: 10.5923/j.ajmms.20241411.47.
1. Introduction
The number of people on programmed hemodialysis is increasing in the world and also in Uzbekistan, probably due to new achievements in the diseases treatment and accessibility of hemodialysis, also due to increasing of people life longevity. Chronic kidney disease (CKD) often futures and consist about 10-17% of total population and yearly growth is about 0.6-1.1% [4,13,15]. Kidney replacement therapy in terminal study of CKD by hemodialysis is necessary life saving treatment [9,13]. Hemodialysis is a step between terminal CKD and kidney transplantation. People who needs in kidney transplantation in the world is vary between 4.9 and 7.083 million according to data from the literature [7,9,13]. Well known that hemodialysis is a special procedure of cleaning the blood from wastes by machine. During cleaning procedures blood minerals also were removed unpredictable manner with development of complications such as cardiac, bone, brain, gastrointestinal due to abnormal metabolism in the whole body [7,9,13]. Abnormal level of parathyroid hormone (PTH), vitamin D high risk to development of secondary hyperparathyroidism (SHPT) [1]. One of the milestones in curing of people on programmed hemodialysis is development of SHPT [6,7,12].In pathogenesis of SHPT main role were shown in decreasing of phosphorus filtration by kidney due to reduced glomerular filtration rate (GFR), increasing its concentration in the blood with further respond to increasing of PHT secretion, decreasing of calcitriol, decreasing of intestinal calcium absorption leads to resorption of calcium from the bone, result of osteoporosis [1,6,7,10]. Long duration of that circle can trigger hyperplasia and adenomas of parathyroid glands [3]. About 5-10% of that people undergoes to parathyroidectomy which is alone treatment for SHPT [12]. SHPT is a risk factor for mortality in patients on programmed hemodialysis [1,3,6,8,15]. Even SHPT is developed in 20-56% patients on programmed hemodialysis not always early detected, even still not recognized [1,6]. Moreover, people on programmed hemodialysis not always by the same reason. In most cases people on programmed hemodialysis the patients with Diabetes Mellitus (DM) with end stages of kidney diseases (ESKD) and estimated more than half of them. Most people with Chronic glomerulonephritis (CGN), gout, chronic pielonephritis and other diseases can develop kidney insufficiency following treatment on programmed hemodialysis. All forms of kidney insufficiency can develop Secondary Hyper parathyroidism (SHPT). The main aim of that study were investigation and compare the cases of increasing the level of PTH in blood plasma in people on programmed hemodialysis.
2. Materials and Methods
Observation were done in Republican Specialized Scientific Practical Medical Centre of Endocrinology named after academiccian Y.Kh. Turakulov and Republican Specialized Scientific Practical Medical Centre of Nephrology and Kidney Transplantation during 2016-2020 by gathering and analyzing data from patient cards whose were admitted into dialysis unit. In total, 465 patient cards were analyzed, and patients were observed. 268 male and 200 female patients among them. We collected data about age, gender, family history, diagnosis, duration of disease, also duration of programmed hemodialysis, total blood count, blood biochemistry included glycemia, HbA1c, AlT, AsT, creatinie, blood urea nitrogen, PTH from patients chart.
3. Results and Discussion
Mean age of patients in the group were significantly different and were younger in CGN group than in group others by 1.19 times, p<0.05 and in DM group by 1.27 times, P<0.05. Average age of patients shown that most of them were in the age between 50-69 years old. That patients distribution and in age probably were related with random observation of cards at those time in the centers. Interestingly, by number 51% of patients presented by people with CGN, 34% with DM, and 15% were on programmed hemodialysis by other reason (Table 1).Table 1. Clinical characteristics of observed patients
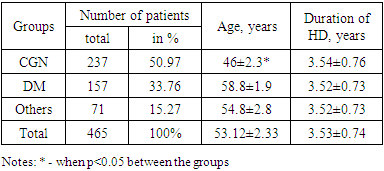 |
| |
|
Whereas, duration of time on programmed hemodialysis were comparable between the groups and were 3.53 years in average.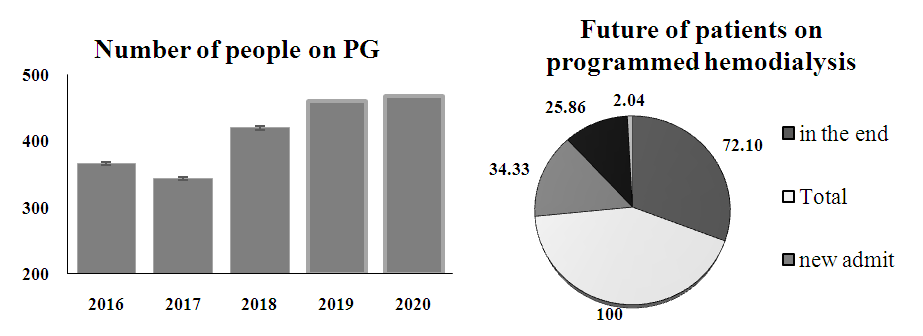 | Figure 1. Number of people on Programmed Hemodialysis by years (A); Future of patients on PG (B) |
Well known that CKD accompanied with anemia due to intoxication and decreasing the erythropoetine secretion [9,12,13]. Total red blood cells were decreased in all groups and were 83.13±5.0, 86.87±3.17 and 88.28±3.58 mg/mL in group of patients with CGN, DM, and others respectively and suggested about severe anemia in those patients [9,16].Blood glucose level were significantly increased in patients with DM (fig.2, А) and were higher than CGN group by 2.4 рtimes, p<0.01 and then group others by 2.05 times, p<0.01. High glycemia level suggested about poor glycemic control in DM group. Blood creatinine level is a waste product of muscle and protein metabolism reflected GFR and presented one of the criterion of CKD severity [1,15]. Blood creatinine level were significantly increased then reference ranges in all patients, more pronounced in CGN group than DM by 1.37 times, p<0.01 and were still higher in other group (fig.2). 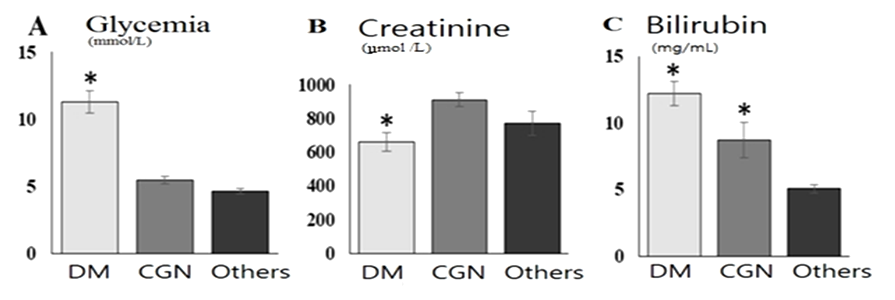 | Figure 2. Blood plasma glucose (А), creatinin (Б) and bilirubin (В) level in patients on programmed hemodialysis. Notes: * - when p<0.05 between compared groups |
Blood bilirubin is a product of break down of red blood cells were more increased in DM group than CGN by 2.14 times, p<0.05 but not in group others (1.4 times, p>0.05), and probably related with more deeper metabolic changes and tissues damage in DM than CKD by other reason, also due to intoxication and decreased GFR [16,18].Blood plasma calcium and phosphorus level in observed patients were higher than reference ranges and did not differs between the groups (fig.3), and suggested about severe mineral abnormalities in CKD due to decreased GFR. In other hands, increasing of calcium and phosphorus level is a trigger mechanism of SHPT development [5,11,14,15,17]. 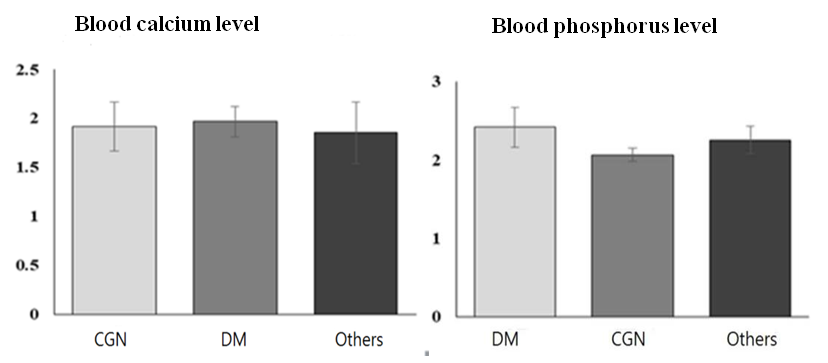 | Figure 3. Blood plasma Calcium and Phosphorus level in observed patients |
Hyperphosphatemia stimulates excess of PTH secretion, together with low vitamin D prevented intestinal calcium absorption and leads to osteoporosis [14]. Chronic hyper phosphatemia and low calcium in patients on programmed hemodialysis leads to development of hyperplasia and parathyroid adenomas [5,6,16].Blood serum PTH level were measured in 15% of observed patients on programmed hemodialysis only in whose were suspected to SHPT, but not in all patients and indicates about low awareness (fig.4, А).Blood serum PTH level reference range in our lab varies between 15 and 65 pg/ml. Increased level of PTH were detected in 48% on programmed hemodialysis, in other 52% hormone level was in normal ranges. High number of people with increased PTH level probably related with selectively measure of hormone only in people who suspected, i.e. have some clinical or biochemical signs of SHPT (fig.4, B). Instead, measure hormone level in all people on programmed hemodialysis probably helps to early diagnosis and start to prevention of parathyroid adenomas. 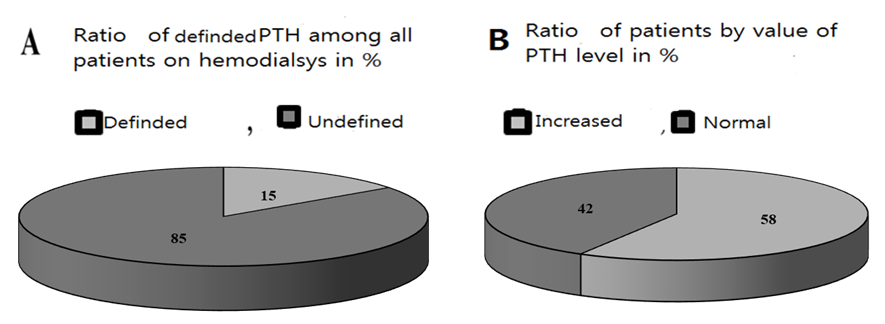 | Figure 4. Ratio of patients whose blood plasma PTH level were measured (A); Ratio of patients with increased and normal level of blood PTH level among those 15% (B) |
Distribution of patients inside the groups by PTH level under 200 pg/mL and higher shown that data were comparable. Indeed despite the reason for CKD the main problem here is decreased GFR (fig.5) in patients on programmed hemodialysis [1,3,6,7]. Data from literature showed that SHPT development had correlation with death rate in people on programmed hemodialysis [1,6,14,15]. Since our patients have been treated on programmed hemodialysis more than 3 years in average, we tried to calculate the risk of hyperparathyroidism and SHPT in the groups.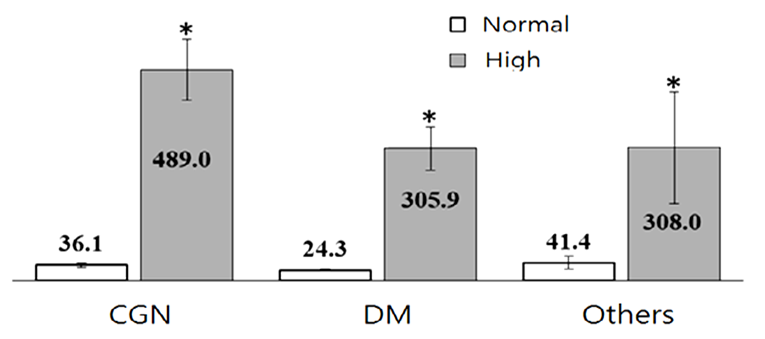 | Figure 5. Averages of normal and increased blood PTH level by groups |
Our results showed that risk for SHPT were high and presented about 50% among those who were tested for blood PTH level. (table 1). Distribution of high blood serum PTH level between the groups (Figure 5) showed high prevalence of hyperparathyroidism state in CGN group (65%), then in DM (30%) and others (5%). Estimation of the relative risk of SHPT in people on programmed hemodialysis suggested a higher risk in DM and other groups than in CGN (Fig.6). 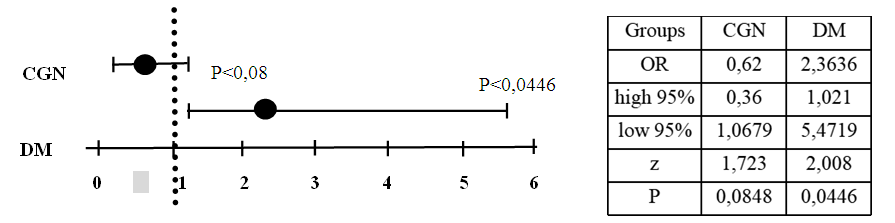 | Figure 6. Odds Ratio and 95% CI risk of SHPT in patients on programmed hemodialysis |
Since group Other was composed of people on programmed hemodialysis for different reasons and data could not be accurate, we calculated the relative risk in Odds Ratio of SHPT in patients on programmed hemodialysis (Fig.6). Significant value of Odds Ratio and 95% CI were shown in DM group (2.36 vs 0.62) and suggested about high risk of SHPT, which probably related with higher level of metabolic disturbances in hyperglycemia state. In conclusion, blood PTH level were measured only in those who were suspected to SHPT and consist 15% of patients on programmed hemodialysis and suggested about low awareness. Among these 15% patients increased blood PTH level were found almost in half (48%). That results suggested about high risk of SHPT among patients on programmed hemodialysis, especially with DM and need to be frequently screened to SHPT by measure blood serum PTH level.
References
| [1] | Copland M, Komenda P, Weinhandl ED, McCullough PA, Morfin JA. Intensive Hemodialysis, Mineral and Bone Disorder, and Phosphate Binder Use. Am J Kidney Dis. 2016 Nov; 68(5S1): S24-S32. doi: 10.1053/j.ajkd.2016.05.024. PMID: 27772640. |
| [2] | DE FRANCISCO A.L.M., COBO M.A, MARIA A. SETIEN Effect of serum phosphate on parathyroid hormone secretion during hemodialysisKidney International, Vol. 54 (1998), pp. 2140–2145. |
| [3] | Murray S.L. and Wolf M. Calcium and Phosphate Disorders: Core Curriculum Am J Kidney Dis. 83(2): 241-256. doi: 10.1053/ j.ajkd.2023.04.017. |
| [4] | Patel L, Bernard LM, Elder GJ. Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials. Clin J Am Soc Nephrol. 2016 Feb 5; 11(2): 232-44. doi: 10.2215/CJN.06800615. Epub 2015 Dec 14. PMID: 26668024; PMCID: PMC4741042. |
| [5] | Leaf DE, Wolf M. A physiologic-based approach to the evaluation of a patient with hyperphosphatemia. Am J Kidney Dis. 2013; 61: 330-336. https://doi.org/10.1053/j.ajkd.2012.06.026. |
| [6] | Ramakrishnan K, Braunhofer P, Newsome B, Lubeck D, Wang S, Deuson J, Claxton AJ. The economic impact of improving phosphate binder therapy adherence and attainment of guideline phosphorus goals in hemodialysis patients: a Medicare cost-offset model. Adv Ther. 2014 Dec; 31(12): 1272-86. doi: 10.1007/s12325-014-0170-4. Epub 2014 Dec 6. PMID: 25479935. |
| [7] | Cannata‑Andía JB., Martín‑Carro B, Martín‑Vírgala1 J, Rodríguez‑Carrio J, Bande‑Fernández JJ, Alonso‑Montes C, Carrillo‑López. Chronic Kidney Disease—Mineral and Bone Disorders: Pathogenesis and Management. Calcified Tissue International (2021) 108: 410–422 https://doi.org/10.1007/s00223-020-00777-1. |
| [8] | Coen G., Manni M., Mantella D. et al. Are PTH serum levels predictive of coronary calcifications in haemodialysis patients? // Nephrol. Dial. Transplant. 2007. Vol. 22. № 11. P. 3262–3267. |
| [9] | Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third national health and nutrition examination survey. Am J Kidney Dis. 2017; 41(1): 1-12. doi:10.1053/ajkd.2003.50007. |
| [10] | Danese M., Belozeroff V., Smirnakis K. et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008. 3. Pp. 1423-1429. |
| [11] | Floege J., Kim J., Ireland E. et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol. Dial. Transplant. 2010. 10. Pp. 1-8. |
| [12] | Guideline Working Group, Japanese Society for Dialysis TherapyClinical Practice Guideline for the Management of Secondary Hyperparathyroidism in Chronic Dialysis Patients. Therapeutic Apheresis and Dialysis 12(6): 514–525. doi: 10.1111/j.1744-9987.2008.00648.x. |
| [13] | Ji-Cheng LV, Zhang Lu-Xia. Prevalence and Disease Burden of Chronic Kidney Disease. Adv Exp Med Biol. 2019; 1165: 3-15. doi: 10.1007/978-981-13-8871-2_1. |
| [14] | KDIGO clinical practice for the diagnosis, evaluation, prevention and treatment of Chronic Kidney Disease-Mineral and Bone Disease (CKD-MBD) // Kidney Int. Suppl. 2009. Vol. 113. P. 1–130. |
| [15] | Li J, Liu D and Liu Z (2021) Serum Total Bilirubin and Progression of Chronic Kidney Disease and Mortality: A Systematic Review and Meta-Analysis. Front. Med. 7: 549. doi: 10.3389/fmed.2020.00549. |
| [16] | Majoni SW, Barzi F, Hoy W, MacIsaac RJ., Cass A, Maple-Brown L, Hughes JT. Baseline liver function tests and full blood count indices and their association with progression of chronic kidney disease and renal outcomes in Aboriginal and Torres Strait Islander people: the eGFR follow- up study. BMC Nephrology (2020) 21: 523-533. |
| [17] | Rothmund M, Wagner PK, SchaA C: Subtotal Parathyroidectomy versus total Parathyroidectomy and auto-transplancation in secondary hyperparathyroidism; a randomized trial. Worid J Sui^rg. 15: 745-750, 1991. 5. Higgins RM, Richardson AJ, Ratcliffe PJ, et al: Total Parathyroidectomy alone or with autograft for renal hyperparathyroidism? Q J Med. 79: 323-332, 1991. |
| [18] | Wang J., Wang B., Liang M. et al. (2017) Independent and combined effect of bilirubin and smoking on the progression of chronic kidney disease. Clin. Epidemiol., 10: 121–132. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML