Sadikova A. M.
Tashkent Pediatric Medical Institute, Uzbekistan
Correspondence to: Sadikova A. M., Tashkent Pediatric Medical Institute, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Juvenile idiopathic arthritis (JIA) is a common chronic rheumatic disease in children, which is a probable cause of early disability. This study provides a detailed analysis of the main difficulties in the classification of JIA and new approaches to the study of the molecular mechanisms of the disease. Juvenile idiopathic arthritis (JIA) is a common type of chronic rheumatoid disease affecting children under 18 years of age and is one of the important causes of early disability [1]. Epidemiological studies have shown that the incidence of JIA ranges from 1.6 to 23 per 100,000 children per year, and the prevalence of JIA is 3.8–400 per 100,000 children in Europe. Girls have a higher incidence rate than boys (10.0 per 100,000 versus 5.7 per 100,000) [1-3]. The negative impact of JIA on the physical and mental development of children seriously affects the quality of life of affected children, causing joint pain, physical disability, anxiety and depression [4-7]. There are no pathogenetic drugs for JIA yet. However, JIA requires intensive treatment to control its development and symptoms. For this severe disease with an unclear etiology, it should be noted that it is necessary to understand the underlying molecular mechanisms, which are currently being determined using new high-tech approaches, primarily due to the development of high-performance omics technologies.
Keywords:
Juvenile idiopathic arthritis
Cite this paper: Sadikova A. M., Studies in Children with Juvenile Rheumatoid Arthritis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2866-2869. doi: 10.5923/j.ajmms.20241411.40.
1. The Aim of the Study
To consider the difficulties of classifying JIA given its unclear etiology. To compare with the classification of arthritis in adults. To find a solution to the problems using molecular genetics methods.
2. The Material of This Study
106 children with juvenile rheumatoid arthritis aged from 3 to 18 years (10.5 ± 1.23), who were undergoing inpatient examination and treatment in the Department of Cardiorheumatology of the RSNPMCP clinic from 2021 to 2023. All patients underwent a complete clinical examination, including laboratory and instrumental studies. As a comparison group, 40 children of the same age were examined. Study results JIA is a group of diseases that are very heterogeneous in etiology and clinical picture. Currently, it is divided into seven subtypes in accordance with the International League of Associations of Rheumatology (ILAR) Pediatrics Working Group [8], including systemic arthritis, oligoarthritis, RF-negative polyarthritis, RF-positive polyarthritis, psoriatic arthritis, enthesitis-associated arthritis and undifferentiated arthritis [9]. Oligoarthritis is the most common category of JIA, accounting for 50–60% of all cases; the frequencies of other subtypes are: polyarthritis 30–35%, systemic JIA 10–20%, psoriatic arthritis 2–15%, and enthesitis-associated arthritis 1–7% [10]. After 25 years of clinical application of the ILAR classification, it is now clear that some JIA subtypes are quite heterogeneous, such as RF-negative polyarthritis and psoriatic subtypes [10]. Ambiguity in the classification of some patients has also been a problem. Clinical efforts to revise the ILAR classification are currently underway [11]. The ILAR subtypes are a work in progress and their shortcomings are now widely recognized. These include complex and often contradictory inclusion and exclusion criteria; changing subtypes in patients followed over time; and growing evidence that the ILAR boundaries do not reflect the underlying disease biology. In fact, the age cutoff of 18 years is defined without any pathophysiological or epidemiological basis and represents a gaping nomenclature gap that separates pediatric and adult rheumatology [12]. With the number of new treatments now outstripping the availability of patients for clinical trials, it is now more important than ever to define biological subgroups within childhood arthritis to identify patients who will benefit from targeting a specific disease mechanism, given the results obtained in arthritis patients worldwide. Age cutoffs should not be applied due to the imprecision of nomenclature across the entire age range. The challenge now is to more accurately define the characteristics of patient subgroups. Recent attempts to address this complex issue have used clinical and blood biomarkers, morphologic characteristics, and informed expert opinion [13]. However, such studies are limited to pediatric patients and face the additional problem that individual processes may produce convergent clinical phenotypes. For example, seronegative and seropositive adult rheumatoid arthritis (RA) show considerable clinical overlap despite fundamental differences in the roles of autoantibodies, complement, and synovial T cells [14]. Phenotypic convergence has similarly been illustrated in animal models of arthritis, in which similar clinical features may manifest in very different ways. Thus, although patient phenotyping should be noted to be useful for subgroup definition and will indeed be required for clinical practice, additional approaches remain necessary. One such approach is genetic studies.In terms of the study, the percentage of age groups of children in our survey is as follows: children aged 3 to 7 years accounted for 12.3% of the total, which amounted to 13 children; children aged 7 to 14 years accounted for 55.7% of the total, which amounted to 59 children; those aged 14 years and older represented 32.1% of the total, which amounted to 34 children.Table 1. Age distribution of children with juvenile rheumatoid arthritis (%)
 |
| |
|
In the process of analyzing the gender component, it was found that there is a slight advantage of the male sex - 51.9% of the total, while the female sex was 48.1%.The diagnosis was determined on the basis of clinical and functional data in accordance with the international consensus on the diagnosis and treatment of rheumatic diseases. (ICD-10). The diagnoses were verified on the basis of a thorough collection of anamnesis, clinical, laboratory (general blood test, urine test) biochemical blood tests, instrumental (radiography, electrocardiography, ultrasound). Particular attention was paid to the duration of the pathological process, past and concomitant diseases.During the clinical and radiological examination of children, the following indicators were determined: the volume and localization of bone and joint damage; characteristics of joint damage (deformation without their significant expansion, type and localization); period of the disease (exacerbation; remission).The patients were observed in a hospital and in a consultative dispensary office.The clinical and anamnestic features of the course of the diseases of the 106 patients examined were clarified through their own observations, medical documentation data, namely the clinical history of the disease, data from the conclusions of narrow specialists, as well as through a questionnaire.All observed children were examined using generally accepted clinical, laboratory and instrumental methods. To establish a diagnosis, all patients underwent the following laboratory and instrumental research methods in 100% of cases: general blood and urine tests upon admission to the hospital and upon discharge; biochemical blood test, necessarily including the determination of CRP, ASLO, RF, total protein, creatinine, urea, ALT, AST, bilirubin; immunogenetic indicators. Instrumental research methods: radiography; consultation of related specialists to clarify the presence of concomitant pathology. Immunological research methods: assessment of the immune status according to modern concepts implies a comprehensive study of the immune system, including testing of its most important functional links. Determination of cytokine status: to determine the concentration of IL-8, IL-17A, IFNγ in the blood serum of the study groups, a three-stage "sandwich" method was used - this is a type of three-phase ELISA. This method uses monoclonal antibodies and cytokines, consisting of 3 stages. First stage: Samples (test and control) are incubated in wells with antibodies, which are immobilized. Then the cytokines of the samples bind to the antibodies. In the second stage, the incubation process is accompanied by a reaction with conjugate No. 1 (human cytokine antibodies with biotin). Third stage: During incubation, the formed biotinylated cytokine antibodies are combined with horseradish peroxidase with streptavidin (conjugate No. 2). The amount of bound conjugate No. 2 is determined using a color reaction. In this case, it is mixed with the horseradish peroxidase substrate (hydrogen peroxide) and a chromogen (tetramethylbenzidine). The intensity of the coloring is determined by the amount of cytokines present in the sample.Determination of C-reactive protein (CRP): the concentration of C-reactive protein (CRP) was determined in the blood serum using the ELISA method according to the attached instructions. The Cytokine test kit was used.Determination of lactoferrin level: the method used is based on a two-site solid-phase enzyme immunoassay. The analysis is performed in two stages. In the first stage, calibration samples with a known concentration of lactoferrin, as well as the samples to be tested, are incubated in the wells of a strip plate with highly specific antibodies to lactoferrin immobilized on the surface of the wells. In the second stage, lactoferrin bound to the antibodies in the wells is treated with a conjugate of antibodies to lactoferrin with horseradish peroxidase. After removing the excess conjugate, a substrate mixture for peroxidase containing hydrogen peroxide o-phenylenediamine is added to the wells and the "Antigen - lactoferrin - conjugate" complex is detected. In this case, o-phenylenediamine is oxidized to form a dye, the intensity of which is proportional to the concentration of lactoferrin in the analyzed sample. The enzymatic reaction was stopped by adding a stop reagent, and the color intensity was measured on an ELISA photometer at 492 nm. (Vector Best test system). Molecular genetic research methods: sample preparation and DNA extraction. Peripheral blood samples: venous blood from the cubital vein (3-5 ml) was used for DNA extraction (vacutainers were used for this purpose) with an anticoagulant/preservative of 15% tripotassium EDTA (Ethilen dianin-tetra acetic acid). A two-stage blood cell lysis method was used to obtain genomic DNA. Red blood cells were destroyed by double centrifugation of the entire volume of whole blood in RCLB buffer (Red cells lyses buffer) at 1500 rpm for 15-20 minutes. The use of RCLB leads to osmotic shock of erythrocytes, leading to their swelling and further destruction. The supernatant containing the destroyed erythrocytes was carefully drained from the tube and the residue above the sediment was sucked off. The clot of leukocyte mixture remaining at the bottom was lysed in WCLB (White cells lyses buffer) in an amount depending on the volume of the leukocyte mixture. WCLB simultaneously serves as a preservative (even at room temperature) for storing leukocyte mass lysates and in this form the lysates can be stored indefinitely. Using the alcohol-salt treatment method, further purification of leukocyte mass lysates was carried out (S. Miller et al., 1988) in a modernized form proposed by the Human Genomics Laboratory of the Institute of Immunology of the Academy of Sciences of the Republic of Uzbekistan (currently the Department of Genomic and Cellular Technologies of the Russian Scientific Center of Immunology of the Ministry of Health of the Republic of Uzbekistan). After the alcohol had dried, a diluted TE (Tris-EDTA) solution 1:3 (TE: water) pH 8.0 was added to the test tube with the dried DNA.Real-time DNA sequence analysis based on Q-PCR HRM technology.Study of the association of IL-17A gene polymorphism with juvenile rheumatoid arthritis in the Uzbek population: to date, there are a lot of often conflicting data on the polymorphism of cytokine genes. Thus, there is evidence that in populations of people living in different countries, the same mutations can lead to different consequences. For example, it has been shown that nucleotide polymorphism in the rs2275913 allele is a likely cause of being associated with the risk of developing rheumatoid arthritis in Mexico, however, such a correlation was not found in Poland. Statistical analysis of fifteen different studies showed that nucleotide polymorphism in this position is a probable cause of being associated with a reduced risk of developing rheumatoid arthritis [3].At present, it is completely unclear what the reason for such contradictory information is, and what role differences in populations of people from different countries or the size of the patient sample in each specific case play.Based on the above, we conducted a molecular genetic study of the association of IL-17A gene polymorphism in children of Uzbek nationality.In this study, we studied the nature of the distribution of allele frequencies and genotypes of the G-197A polymorphic variant of the IL-17A gene in children with JRA.Table 2. Frequency of occurrence of IL-17A (G-197A) polymorphism in groups with JRA and healthy controls
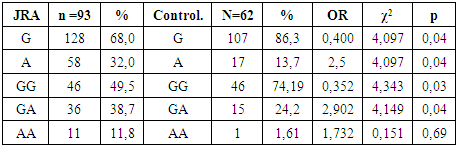 |
| |
|
As can be seen from our results, the risk marker for the development of JRA is the A allele and the homozygous AA genotype (32.0% and 11.8%, respectively; OR = 1.732; 95% CI: 0.937> 3.273> 17.781; χ2=0.151 (p=0.05)). Further, when comparing the GG genotypes, reliable differences were revealed between children with JRA and the control group (49.5% and 74.19%, respectively; OR = 0.352; 95% CI: 0.307> 0.508> 0.971; χ2=4.343 (p=0.03)). When analyzing the heterozygous GA genotype, differences were also found between the frequency of occurrence in children with JRA and the control group (38.7% and 24.2%, respectively; OR = 2.902; 95% CI: 0.877> 1.764> 3.549; χ2 = 4.149). The AA genotype was more common in the group with JRA compared to the healthy group, but this indicator was not significant (OR = 1.732, χ2 = 0.151, Wald 95% CI: 0.105> 1.732> 28.43). Conclusions: The data obtained in this study on the distribution of frequencies of allelic variants, genotypes and association of the IL-17A cytokine gene (G197A) with juvenile rheumatoid arthritis can be used as marker features of the genetic characteristics of this pathological condition.Thus, a reliable increase in the A allele and homozygous genotype AA confirms the involvement of systemic inflammatory reactions in the pathogenesis of such a disease as JRA.The study emphasizes the need to revise existing classifications of JIA and use molecular genetic methods to more accurately determine subgroups of patients.
References
| [1] | Abramkin AA, Lisitsyna TA, Veltishchev DYu, Seravina OF, Kovalevskaya OB, Nasonov EL. The influence of synthetic disease-modifying anti-inflammatory drugs, genetically engineered biological agents and psychopharmacological therapy on the dynamics of mental disorders in patients with rheumatoid arthritis // Scientific and practical rheumatology. 2017 - No. 55 - Vol. 4 - P. 393-402. |
| [2] | Avdeeva AS, Artyukhov AS, Dashinimaev EB, Cherkasova MV, Nasonov EL. Dynamics of cytokine profile parameters against the background of the use of a biosimilar of rituximab (Acellbia, BIOCAD) and the original drug (MabThera, F. Hoffmann-La Roche Ltd., Switzerland) in the treatment of rheumatoid arthritis // Scientific and practical rheumatology. 2019 - No. 57 - Vol. 1 -P. 46-55. |
| [3] | Avdeeva AS, Cherkasova MV, Kusevich DA, Rybakova VV, Nasonov EL. Immunological effects of a biosimilar of rituximab (Acellbia, BIOCAD) in patients with rheumatoid arthritis // Scientific and practical rheumatology. 2018 - No. 56 - Vol. 5 -P. 556-563. |
| [4] | Beketova TV, Arsenyev EV. Interleukin 5 - a new target for the therapy of eosinophilic granulomatosis with polyangiitis // Scientific and practical rheumatology. 2020 - № 58 - Vol. 3 - P. 321-329. |
| [5] | Beketova TV, Blank LM, Lila AM. COVID-19 in a patient with ANCA-associated systemic vasculitis receiving anti-B-cell therapy with rituximab // Scientific and practical rheumatology. 2020 - № 58 - Vol. 4 - P. 456-462. |
| [6] | Beketova TV, Nasonov EL. Innovative methods of treating Takayasu's arthritis in focus on interleukin 6 inhibitors. Own experience of using tocilizumab and literature review // Scientific and practical rheumatology. 2017 - № 55 - Vol.5 - P. 536-548. |
| [7] | Beketova TV, Popov IY, Babak VV. Review of recommendations for the treatment of ANCA-associated systemic vasculitis presented in 2021 by the American College of Rheumatology and the Vasculitis Foundation // Scientific and Practical Rheumatology. 2021 - № 59 - Vol.6 - P. 684-692. |
| [8] | Volokitina, EA Hip arthroplasty for protrusive deformity of the acetabulum against the background of rheumatoid arthritis complicated by a femur fracture (case report) // Ural Medical Journal. - 2018. - No. 8 - Vol.163 - P. 117—121. |
| [9] | Gaidukova I.Z. Comorbidity in inflammatory diseases of the joints and spine - trends of the 21st century. // Ter. archive - 2018. - No. 12 - Vol. 90 - P. 90-95. |
| [10] | Gaidukova I.Z., Rebrov A.P., Korotaeva T.V., Dubinina T.V., Otteva E.N., Badokin V.V., et al. Remission in axial spondyloarthritis - definition and assessment tools // Scientific and practical rheumatology. 2018 - No. 56 - Vol. 1 - P. 10-14. |
| [11] | Gordeev A.V., Olyunin Yu.A., Galushko E.A., et al. Difficult-to-treat rheumatoid arthritis. What is it? // Modern rheumatology. 2021 - No. 15 - Vol. 5 - P. 7-11. |
| [12] | Dubinina TV, Gaidukova IZ, Godzenko AA, Lapshina SA, Rebrov AP, Rumyantseva OA. Recommendations for assessing disease activity and functional state of patients with ankylosing spondylitis in clinical practice // Scientific and practical rheumatology. 2017 - No. 55 - Vol. 4 - P. 344-350. |
| [13] | Eliseev MS, Nasonov EL. Use of canakinumab in gout // Scientific and practical rheumatology. 2018 - No. 56 - P. 41-48. |
| [14] | Eliseev MS. Recommendations of the American College of Rheumatology (2020) for the management of patients with gout: what's new and what's controversial // Scientific and practical rheumatology. 2021 - № 59 - V.2 - P. 129-133. |
| [15] | Mazurov VI, Gaidukova IZ, Erdes Sh, Dubinina TV, Pristrom AM, Kunder EV, et al. Efficacy and safety of netakimab, a monoclonal antibody against interleukin-17A, in patients with active ankylosing spondylitis. Results of an international multicenter randomized double-blind phase III clinical trial BCD-085-5/ASTERA // Scientific and practical rheumatology. 2020 - № 58 - V.4 - P. 376-386. |
| [16] | Makarov S. Yu. Features of the clinical picture of juvenile arthritis in children with connective tissue dysplasia // Bulletin of the Council of Young Scientists and Specialists of the Chelyabinsk Region. 2017. - No. 2 - Vol. 17 - P. 73-77. |
| [17] | Nasonov EL, Avdeeva AS, Lila AM. Efficacy and safety of tofacitinib in immune-mediated inflammatory rheumatic diseases (part I) // Scientific and practical rheumatology. 2020 - No. 58 - Vol. 1 -P. 62-79. |
| [18] | Nasonov EL, Avdeeva AS, Lila AM. Efficacy and safety of tofacitinib in immune-mediated inflammatory rheumatic diseases (part II) // Scientific and practical rheumatology. 2020 - No. 58 - Vol. 2 - P. 214-224. |
| [19] | Nasonov EL, Avdeeva AS, Popkova TV. New Possibilities of Pharmacotherapy of Systemic Lupus Erythematosus Prospects for the Use of Anifrolumab (Monoclonal Antibodies to Interferon Receptors Type I) // Scientific and Practical Rheumatology. 2021 - No. 59 - Vol. 5 - P. 537-546. |
| [20] | Nasonov EL, Avdeeva AS. Depletion of B Cells in Immunoinflammatory Rheumatic Diseases and Coronavirus Disease 2019 (COVID-19) // Scientific and Practical Rheumatology. 2021 - No. 59 - Vol. 4 - P. 384-393. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
