Gafur-Akhunov M. A.1, Pulatova D. Sh.2, Abdikarimov Kh. G.3, Tadjibayev A. A.4, Kasimov U. K.4, Egamberdiev S. K.1, Tursinov I. T.5, Nasirov S. K.6
1Center for the Development of Professional Qualifications of Medical Workers of the Ministry of Health of the Republic of Uzbekistan
2Children's Oncology, Hematology and Immunology Scientific and Practical Medical Center, Tashkent, Uzbekistan
3Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology
4Tashkent Regional Branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology
5Namangan Regional Branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology
6Tashkent Medical Academy, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
An analysis of the effectiveness of treating giant cell tumors of tubular bones was conducted in 462 patients who were treated in oncology clinics of the Republic of Uzbekistan from 2010 to 2022. Of the 462 patients, 254 (54.9%) were men and 208 (45.1%) were women. In most cases, the tumor occurred in the age group of 20-40 years (76.2%). The tumor was most often localized in the epimetaphyseal regions of the femur, tibia, and humerus, less frequently in the bones of the forearm, fibula, small tubular bones of the hand and foot, and in the clavicle. Of the 462 patients, 341 (73.9%) had a benign form, while 121 (26.1%) had a malignant tumor.Of the 462 patients, 10 (2.1%) underwent curettage, 91 (19.7%) underwent curettage with autoplasty, 11 (2.4%) underwent marginal resection, 94 (20.4%) underwent segmental resection, 15 (3.3%) underwent mutilating operations (amputation or disarticulation), 156 (33.8%) underwent curettage with cement augmentation, 38 (8.2%) underwent curettage with cryotherapy and cement augmentation, 35 (7.5%) patients received neoadjuvant targeted therapy + surgery + adjuvant targeted therapy, and 12 (2.6%) patients underwent surgery + adjuvant targeted therapy.The results of the study showed that the highest recurrence rates for giant cell tumors were observed after curettage surgery - 66.7%, with metastases in 33.3%. After marginal resection, recurrence was observed in 33.3% of patients, after curettage with autoplasty - in 20.7%, after segmental resection - in 5.3%, after mutilating operations - in 6.6% with metastases in 6.6%, after curettage with cement augmentation - in 9.6% with metastases in 2.5%, and after curettage with cryotherapy and cement augmentation - in 5.2% with metastases in 2.6%. Following neoadjuvant targeted therapy, surgery, and adjuvant targeted therapy, there was no recurrence or metastasis, while after surgery with adjuvant targeted therapy, tumor recurrence was detected in 8.3% of cases.
Keywords:
Giant cell tumor, Tubular bones, Curettage, Cement augmentation, Recurrence, Metastases, Denosumab, Neoadjuvant targeted therapy, Adjuvant therapy
Cite this paper: Gafur-Akhunov M. A., Pulatova D. Sh., Abdikarimov Kh. G., Tadjibayev A. A., Kasimov U. K., Egamberdiev S. K., Tursinov I. T., Nasirov S. K., Evolution of Treatment for Giant Cell Tumor of Tubular Bones, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2854-2865. doi: 10.5923/j.ajmms.20241411.39.
1. Introduction (Actuality of the Problem)
Giant cell tumor (GCT) is a neoplasm of osteogenic origin that accounts for 15-20% of all benign bone tumors. It most commonly occurs between the ages of 20-40 years. The preferred localization is the tubular bones of the extremities. In 60-70% of cases, the tumor is located in the epiphyses and epimetaphyses of the femur and tibia. In 40% of cases, the tumor affects the entire articular end, causing it to swell, destroying the cortical layer and extending beyond the bone (M.D. Aliev et al., 2018; V.I. Zorya et al., 2012).GCT is classified as an aggressive neoplasm. This highly unpredictable and multifaceted tumor in its clinical course can be benign, primarily malignant, or undergo malignant transformation. Secondary malignant GCT is observed both after non-radical surgical interventions and after inappropriate radiation treatment (G.P. Kotelnikov et al., 2018; E.L. Neishtadt, A.B. Markochev, 2012; R.M. Tikhilov et al., 2017).The vast majority of authors hold the opinion that curettage of the pathological focus should not be used as an independent stage in the treatment of patients with GCT, as this operation is not radical, results in up to 46% recurrence rate, and contributes to malignant transformation of the neoplasm (S.P. Mironov, G.P. Kotelnikov, 2018; M.D. Aliev et al., 2018).Recurrences of GCT are noted in 7-13% of cases even after radical interventions such as segmental resection and resection of the articular end of the radius. * All of this indicates frequent dissatisfaction with the results of surgical treatment in patients with this pathology. (S.V. Dianov 2018; R.M. Tikhilov et al., 2017; A.F. Mavrogenis et al., 2017).In the pathogenesis of GCT, the primary role belongs to the interaction between bone precursor cells and monocytes/macrophages. GCT is characterized by the proliferation of mesenchymal stromal bone precursor cells, which effectively initiate and maintain osteoclastogenesis. The discovery of key molecular signals controlling osteoclast formation was fundamental to understanding the development of bone GCT.Resorbing giant cells are a product of the interaction between stromal cells and monocytes, which subsequently transform into tumor osteoclasts. Multinucleated giant cells constitute the main mass of the tumor, with cellular markers showing a positive reaction to CD45 in multinucleated giant cells, indicating their relationship to monocytes and macrophages. Additionally, hyperexpression is observed in receptors for the ligand of nuclear factor-κB activators (RANKL) and stromal factor SDF-1. Stromal cells often form osteoid. They also secrete various chemokines, including monocyte proteins and SDF-1, which promote migration from the bloodstream into tumor tissue. Monocytes express the receptor activator of nuclear factor kappa-B (RANK), while GCT stromal cells express RANKL, which is necessary for the differentiation and activation of mature osteoclasts. Osteoclast-like multinucleated giant cells resorb bone, leading to bone tissue destruction. Preventing the activation of the single RANKL-RANK receptor on the surface of osteoclasts and their precursors suppresses the process of osteoclast maturation, functioning, and survival, which in turn leads to a decrease in bone resorption and increases bone mass and strength. (Fig. 1)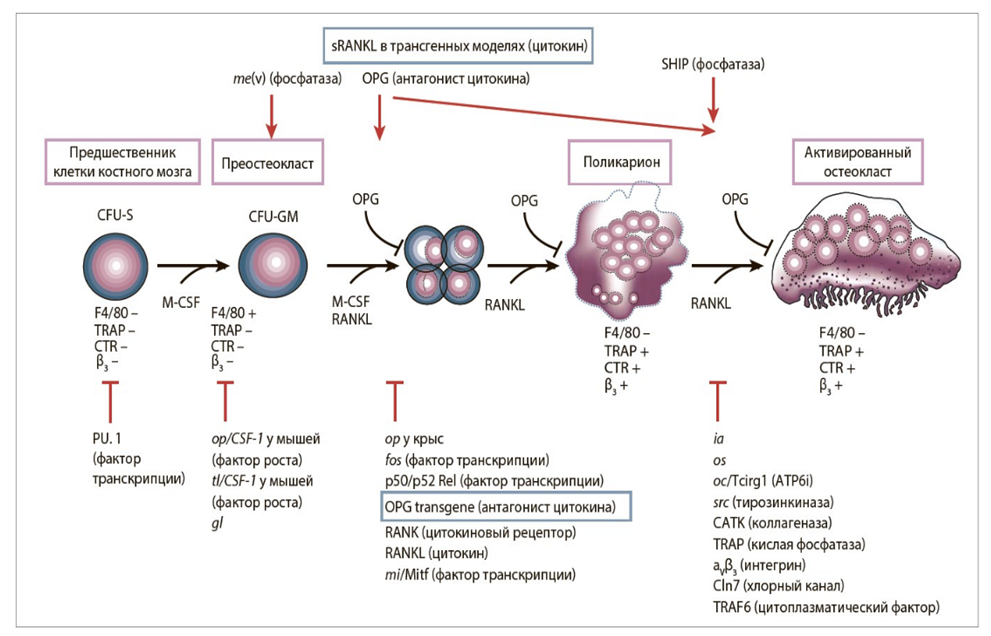 | Figure 1. Pathogenesis of GCT development |
Therefore, giant cell bone tumor is a complex pathology of the bone system, which is characterized by a benign tumor, but in its course and clinical manifestations is considered malignant, and in most cases gives relapses and can metastasize to various organs. Therefore, timely radical surgeries prevent tumor recurrence and metastasis.
2. Materials and Methods of Research
We analyzed the results of treatment of 462 patients diagnosed with giant cell tubular bone tumors in the period from 1986 to 2021, who were treated at the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology, as well as their Tashkent regional, Namangan regional, and Tashkent city branches. Of the 462 patients, 254 (54.9%) were men and 208 (45.1%) were women. The patients' ages ranged from 12 to 56 years, averaging 29.4 years. The majority of patients were aged 20-40 years (76.2%) (Fig. 2).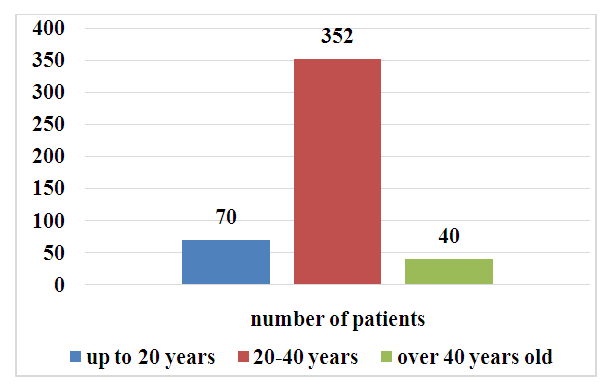 | Figure 2. Distribution of patients by age (n=462) |
Analysis of the tumor localization revealed that in most patients, the giant cell tumor was localized in the epimetaphyseal part of the tubular bones. At the same time, the tumor was most often localized in the distal part of the femoral bones - 164 (35.5%), in the proximal part of the tibia - 133 (28.8%), shoulder bones - 53 (11.5%) and in the bones of the wrist - 50 (10.8%). Relatively rarely in the radial - 20 (4.3%), lamellar - 28 (6.0%), elbow - 9 (1.9%), as well as in the metacarpal - 7 (1.5%) and foot bones - 10 (2.1%) (Fig. 3).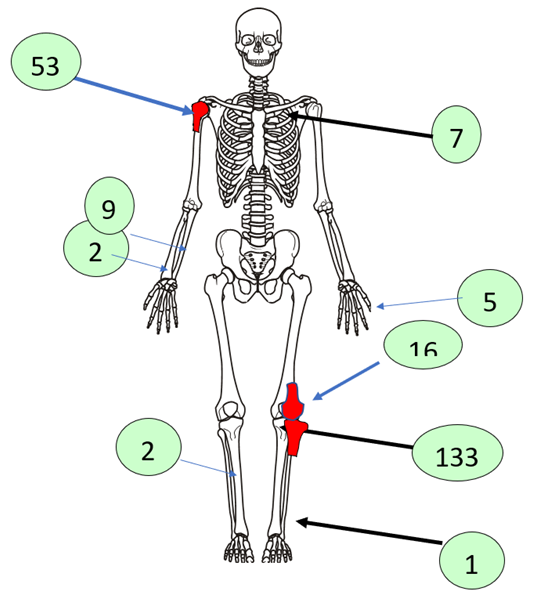 | Figure 3. Tumor localization (n=462) |
Analysis of the clinical symptoms of the disease revealed that out of 462 patients, 400 (86.6%) clinically identified a formation in the projection of the affected bone, 23 (5%) had a tumor, and 39 (8.4%) had a pathological break in the projection of the tumor localization with the presence of pain syndrome, swelling, and limb dysfunction.All patients underwent a comprehensive examination using clinical, laboratory, and instrumental research methods. The radiological method is the leading in the diagnosis of GKD, which was performed on all 462 patients. CT and MSCT scans were performed on 160 patients, MRI scans on 150 patients, and skeletal bone scans on 70 patients. Of the 462 patients examined, 280 (60.6%) had a trabecular-cellular form, 70 (15.2%) had an osteolytic form, and 112 (24.2%) had a mixed form. All patients underwent cytological and histological examinations. The histological structure of the tumor was benign in 341 (73.9%) cases and malignant in 121 (26.1%).Following a comprehensive examination and consensus decision, 462 patients underwent treatment, taking into account the extent of the disease, localization, histological structure, radiological form, and presence of the pathological fracture. In determining the tactics of surgical treatment, the main focus was on the damage to the semicircle of the tubular bones, the bone marrow canal, and the cortical layer, as well as the condition of the soft tissues in the tumor projection. Taking into account these factors and the histological structure of the tumor, treatment tactics were determined.In our observations of 462 patients, 10 (2.1%) underwent excochleation of the tubular bone, 91 (19.7%) underwent excochleation + autoplasty, 11 (2.4%) underwent marginal bone resection, 94 (20.4%) underwent segmental resection, 15 (3.3%) underwent disabling surgeries (amputation or exarticulation), 156 (33.8%) underwent excochleation + cementoplasty, 38 (8.2%) underwent excochleation + cryotherapy + cementoplasty, 35 (7.5%) underwent neoadjuvant targeted therapy + surgery + adjuvant targeted therapy, and 12 (2.6%) underwent surgery + adjuvant targeted therapy.Cementoplasty was performed after the tumor excochleation operation, and the resulting cavity was filled with antibiotic-containing (gentamicin, tauromycin) radiopaque medical bone cement. We used radiopaque sterile medical cement from Johnson & Johnson, DePuy, Stryker, Gentafix, and Simplex, which consisted of polyacrylate with an antibiotic. The medical cement was white, in powder form, available in packages of 20 and 40 g, with a special solvent of 10 and 20 ml. The cement polymerization time under normal conditions (air temperature +20°C, humidity 50%) is 10 minutes.Cryotherapy of the formed cavity was carried out using liquid nitrogen (-196°C). Preliminary measures should be taken to isolate the soft tissues near the operative site with several layers of gauze pads or towels to prevent the refrigerant from contacting the skin and adjacent tissues, which could lead to subsequent necrosis. Despite the availability of modern cryoapplicators, we propose the simplest open method of pouring liquid nitrogen, which was originally described by Marcove and his colleagues. Accordingly, a sized funnel made of stainless steel is inserted into the formed cavity. Liquid nitrogen is poured into the residual cavity and allowed to evaporate, then it is poured again until the cavity temperature reaches -21°C. Simultaneously, soft tissues are continuously irrigated with a warm isotonic solution to protect the skin and adjacent neurovascular tissue from damage. The thawing process lasts 3-5 minutes. This procedure is repeated 3-4 times.Targeted therapy using the drug Prolia (denosumab, Xgeva) was administered in a neoadjuvant regimen at a dose of 120 mg subcutaneously on days 1, 8, 15, and 22 of treatment. After 21 days, the patient returned to the doctor, and the effectiveness of the treatment was assessed (using radiographic and MSCT studies). Adjuvant targeted therapy was conducted with the aforementioned drug once a month with a 28-day interval. Based on the conducted uni- and multi-factor analysis, it was established that when choosing surgical intervention in the form of tumor excochleation, the following features were most significant: tumor growth rate up to 1.0 cm/month; tumor localization in the femur, tibia, and humerus; in the proximal and distal parts of the bone; radiographically - tumor of cellular-trabecular or mixed form; average bone lesion size up to 5 cm; benign tumor variant; absence of soft tissue involvement, cortical layer destruction, joint surface damage, and pathological fracture. The proportion of these combined features ranged from 60 to 100% (Fig. 4).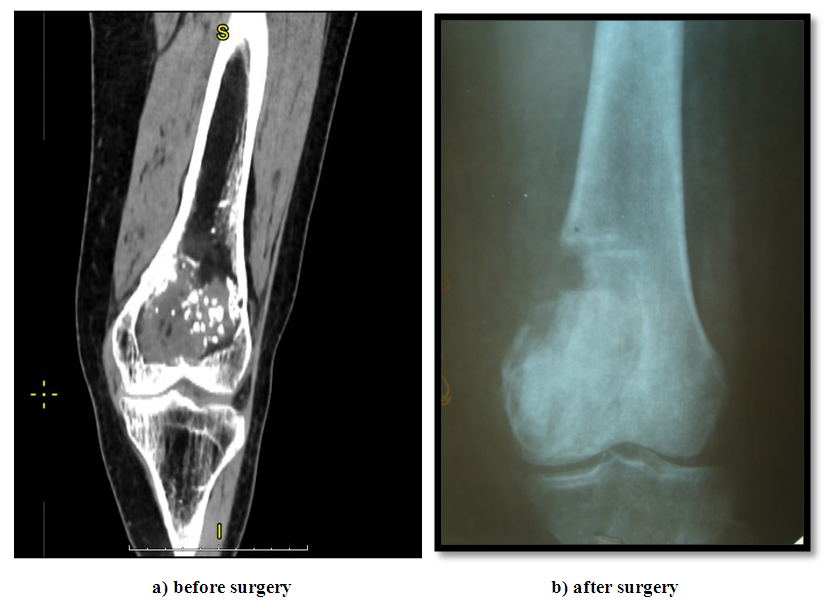 | Figure 4. Excochleation of the femur |
Surgical intervention in the volume of excochleation with autoplasty was most often performed with the following combination of signs: tumor growth rate up to 2 cm/month, tumor localization in the femoral, humerus, shoulder bones and foot bones, in the diaphyseal part of the bone, tumor cellular-trabecular form, with bone size up to 6.8 cm, with benign osteoblastoma variant and absence of damage to soft tissues and cortical layer, as well as destruction of bone, joint surface, one anatomical region and pathological Meanwhile, the frequency of these symptoms ranged from 75 to 100%. All of these specific signs are closely related to the prognosis of tumor recurrence and metastases (Fig. 5).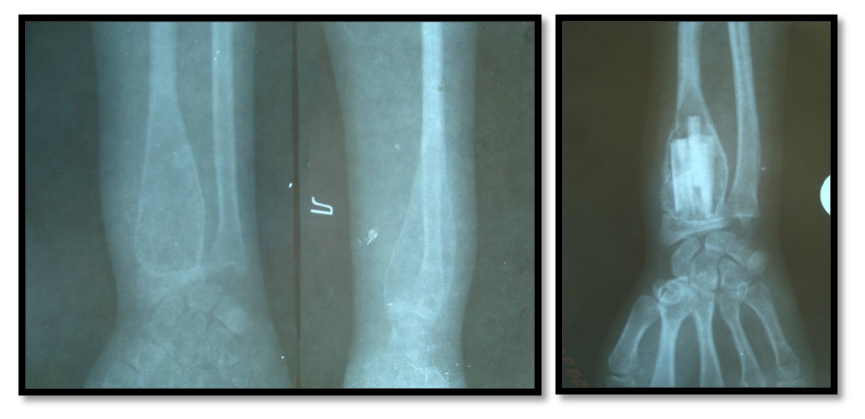 | Figure 5. Excochleation and autoplasty |
Bone marginal resection in giant bone tumors was performed more often with combination of the following features: tumor growth rate from 0.5 to 1.5 cm/month, localization in the femoral, tibial, and wrist bones, in the proximal and distal sections, phalanges and metatarsal bones, radiographically - in the form of a trabecula, with a tumor size of 7.2 cm, histologically - in the form of a benign tumor, with no damage to the soft tissues, partial destruction of the cortical layer, destruction.Segmental resection was most often performed with the combination of the following features: tumor growth rate up to 2 cm/month, localization in the metacarpal, radial bone and spleen, proximal and distal parts of the bone, T1-2N0M0, radiographically - in all tumor forms with a size of 6 to 10 cm, benign and primary - malignant forms, tumor decay, damage to the cortical layer of the bone (partial and complete) and one anatomical region. Meanwhile, the severity of the symptoms ranged from 61.9 to 100% (Fig. 6).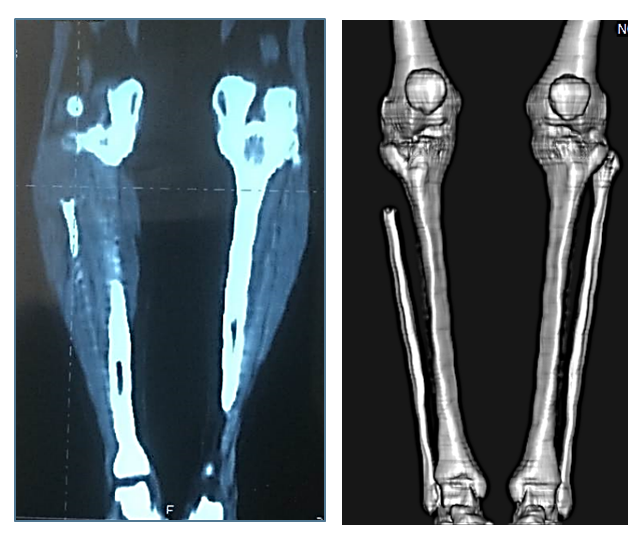 | Figure 6. Segmental resection of the right metacarpal bone |
Segmental resection with autoplasty was most often performed with the presence of the following signs: tumor growth rate from 0.5 to 2 cm/month, tumor localization in the shoulder and lumbar bones. in the proximal and distal parts, with no soft tissue damage, skin and joint movement restrictions, T.). Mo, in all radiological forms, tumor size from 5 to 10 cm. in benign and malignant forms, tumor decay. (macroscopically) partial and complete destruction of the cortical layer and damage to one anatomical area.Meanwhile, the severity of the symptoms ranged from 61.1% to 94%. Segmental resection of the tubular bone with subsequent endoprosthetics was most often performed with the combination of the following features: tumor growth rate from 2.5 to 3.5 cm/month, with its localization in the femoral and shoulder bones, distal and proximal sections, joint movement restriction, in case of T1N0M0 and T2N0M0, radiographically - in the mixed or lytic form, tumor size from 7.5 to 13.5 cm with benign and malignant variants, tumor recurrence, soft tissue lesions, oste Meanwhile, the severity of the symptoms ranged from 55.4% to 88.4% (Figs. 7, 8, 9).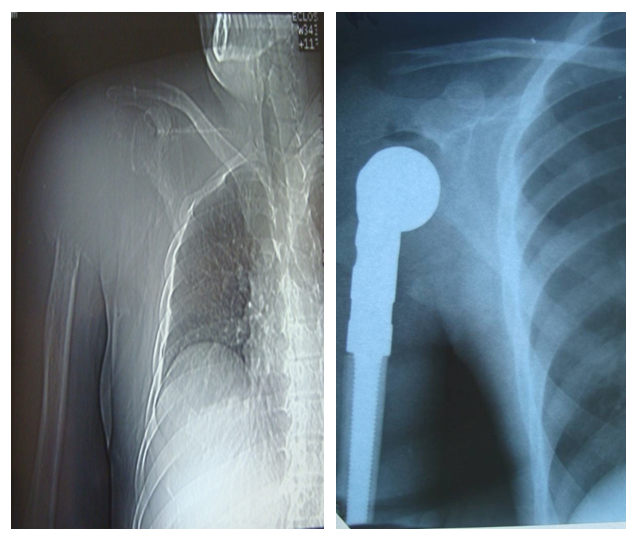 | Figure 7. Segmental resection of the right humerus with endoprosthetics of the humerus joint |
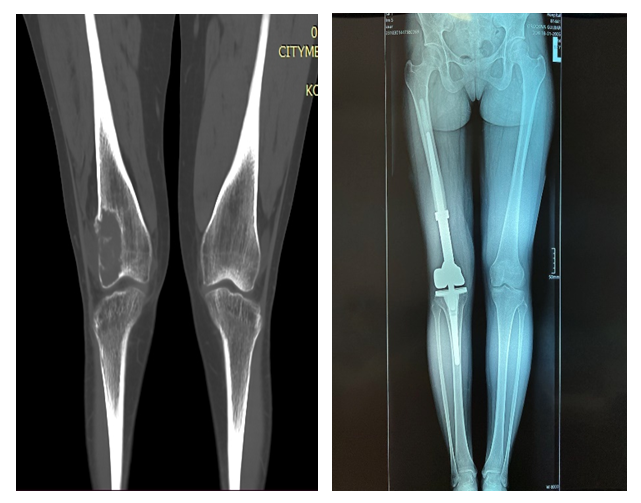 | Figure 8. Segmental resection of the right femur with knee joint arthroplasty |
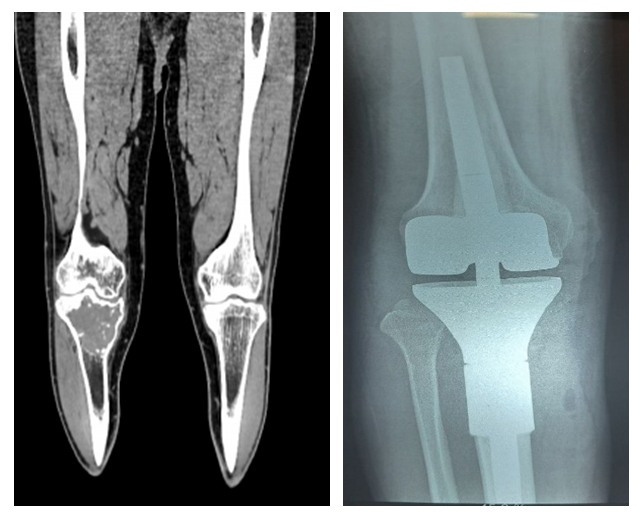 | Figure 9. Segmental resection of the right tibia with knee joint endoprosthesis implantation |
Segmental resection of the tubular bone with compression-distruction osteosynthesis was most often performed with combination of the following signs: tumor growth rate from 2 to 3 cm/month, localization in the femoral and tibial bones, in the proximal and distal parts of the bone, with restriction of movement in the joint, in all histological and radiological forms of the tumor, with its size from 3.5 to 10.5 cm (osteomyelitis), presence of complications after the first operation (penetration of the endoprosthesis, fracture Meanwhile, the severity of the symptoms ranged from 55.6 to 81.9% (Fig. 10).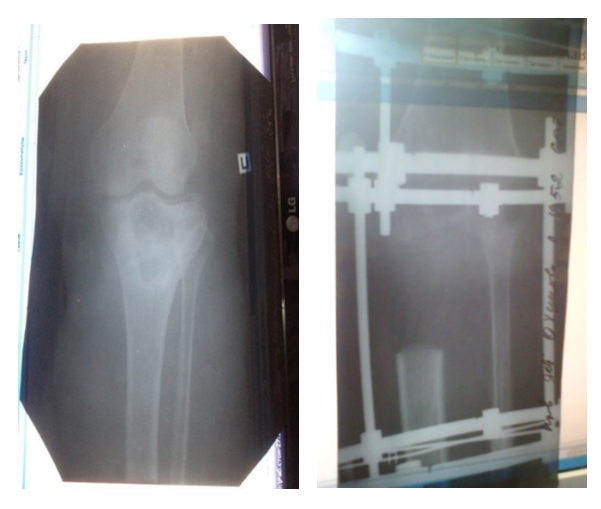 | Figure 10. Segmental resection + compression-distraction osteosynthesis (n=37) |
We performed limb amputation or exarticulation mainly when the following signs were combined: tumor localization in the femoral, tibial, and popliteal bones, with unclear tumor boundaries, joint movement restriction, radiographically mixed or lytic tumor forms, tumor size from 3.5 to 20.5 cm, primary or secondary malignant forms, T1N0M0 and T2N0MO, tumor decomposition (macroscopically), soft tissue damage, complete or partial destruction of the cortical layer, and pathological fracture. Meanwhile, the severity of these symptoms ranged from 53.3% to 93.3%. | Figure 11. Operation: Excochleation + cement plasty |
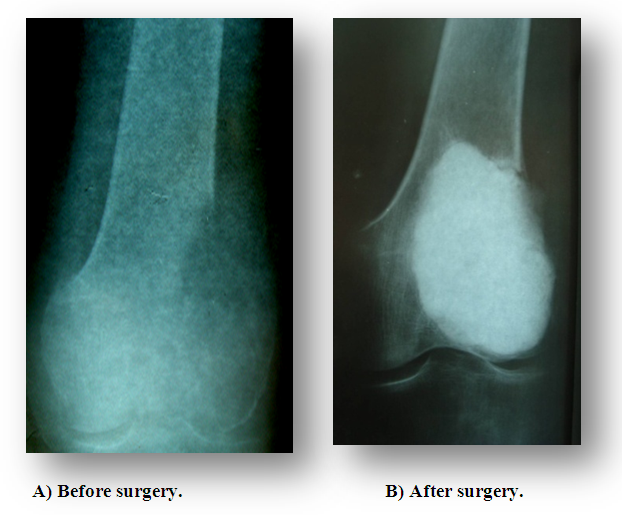 | Figure 12. Excochleation + cryotherapy + cement plasty |
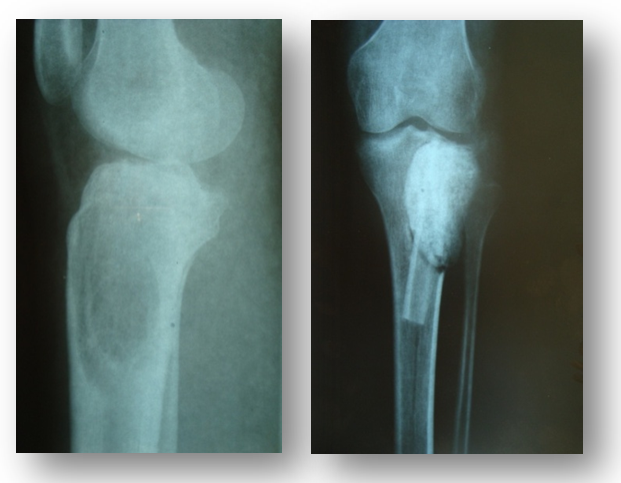 | Figure 13. Patient M., 20 years old. Giant cell tumor of the proximal part of the right tibia. Operation: Excochleation + autoplasty + cementoplasty |
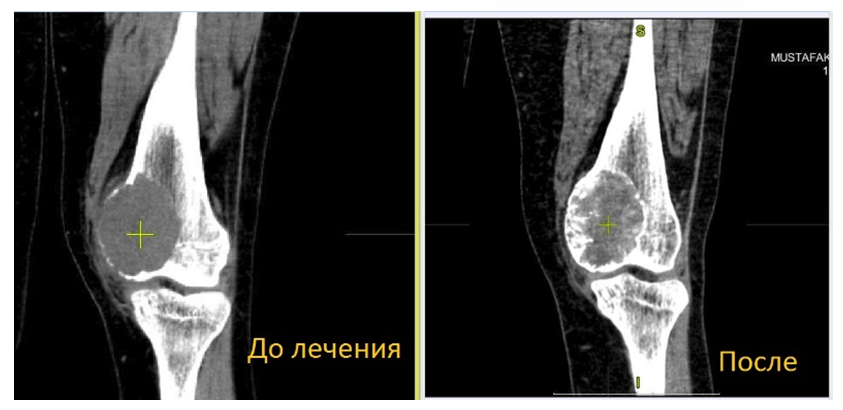 | Figure 14. Neoadjuvant targeted therapy of bone marrow transplantation |
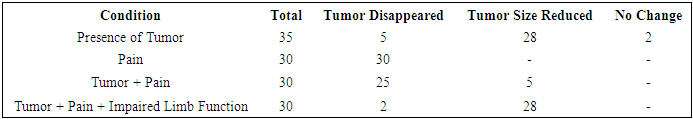
From the data presented in Table 1, it can be seen that the clinical assessment of the effectiveness of the treatment of giant cell tumor (GCC) by Denosumab. Its results showed a decrease in tumor, pain, and limb dysfunction.Table 1. Distribution of Patients by Type of Surgical Intervention for Giant Cell Tumor (GCT)
 |
| |
|
Of the 35 patients, the tumor completely disappeared in 5 (14.3%), the tumor size decreased in 28 (80%), and no changes occurred in 2 (5.7%). In 30 patients, the pain completely disappeared (100%). This indicates the high effectiveness of Denosumab in pain relief. In 25 patients (83.3%), both the tumor and the pain completely disappeared. In 5 patients (16.7%), the tumor size decreased, but the pain disappeared. In 2 patients (6.7%), the tumor, pain, and limb dysfunction completely disappeared. In 28 patients (93.3%), the tumor size decreased, but limb dysfunction persisted.Table 2. Clinical Evaluation of the Effectiveness of Denosumab Treatment for GCT
 |
| |
|
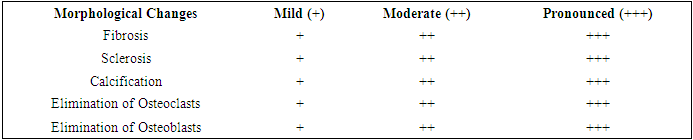
Results show that Denosumab demonstrates high effectiveness in tumor reduction and pain relief in patients with giant cell tumors. However, with a combination of tumor, pain, and limb dysfunction, the improvement is less pronounced.In our observations, to evaluate the morphological characteristics of the tumor in giant cell tumor after neoadjuvant targeted therapy were studied the following histological changes occur: fibrosis, sclerosis, calcification, osteoclast elimination, and osteoblast elimination. However, the main focus is on the severity of these histological changes during microscopic examination. (Table 3)Below is a histological picture of the effectiveness of various options for neoadjuvant targeted therapy with the drug Denosumab 120mg for giant cell bone tumors.Table 3. Morphological Assessment of the Effectiveness of Neoadjuvant Targeted Therapy for Giant Cell Tumor of Bone (GCT)
 |
| |
|
As can be seen from the data presented in Table 3, the low effectiveness of treatment was morphologically characterized by less pronounced changes in fibrosis, sclerosis, calcination, osteoclast elimination, and osteoblast elimination (Fig. 15).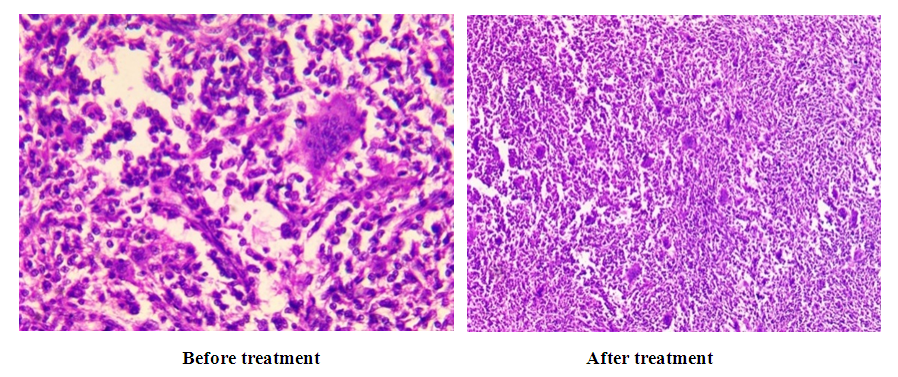 | Figure 15. The morphological picture of the weak effect of bone grafting treatment |
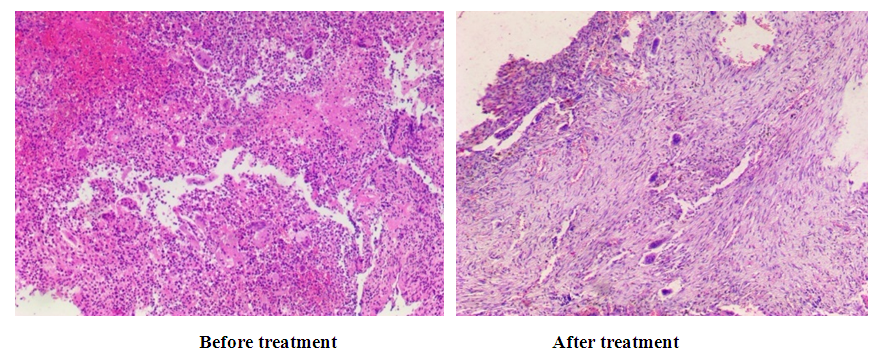 | Figure 16. The morphological picture of the moderate effect of bone grafting treatment |
With moderate effectiveness, these changes were relatively pronounced. With full treatment effectiveness, all of the above-mentioned histological changes were more pronounced. In our observations, these morphological changes were mild in 20% of patients, moderate in 28% and severe in 52% of patients.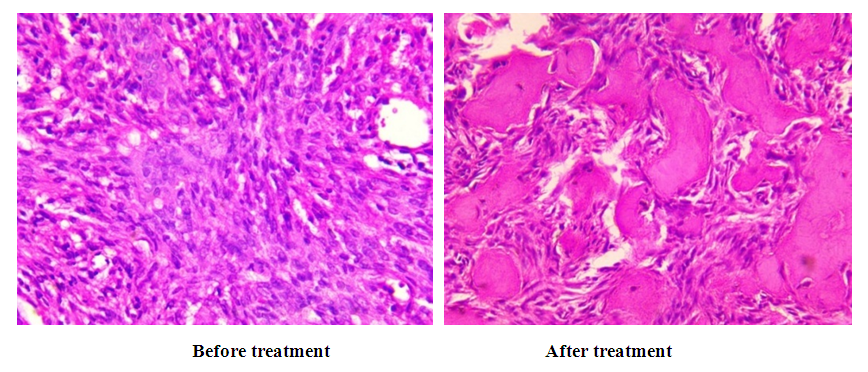 | Figure 17. Morphological picture of the pronounced effect of bone grafting treatment |
Our study comparatively examined the recurrence and metastasis rates after various treatment methods for giant cell tubular bone tumors. It was found that in the group of patients after neoadjuvant targeted therapy (Denosumab 120 mg) according to the standard scheme, surgery and adjuvant targeted therapy, tumor recurrence and metastases were not detected in the observation period up to 3 years. In the group of patients after excochleation and cement plasty, as well as adjuvant targeted therapy (Denosumab 120 mg), tumor recurrence was detected in 8.3% of cases. In the group of patients after excochleation and cement plasty, the frequency of relapses was 9%, and the frequency of metastases was 9% (Table 4).Table 4. Frequency of Tumor Recurrence and Metastasis
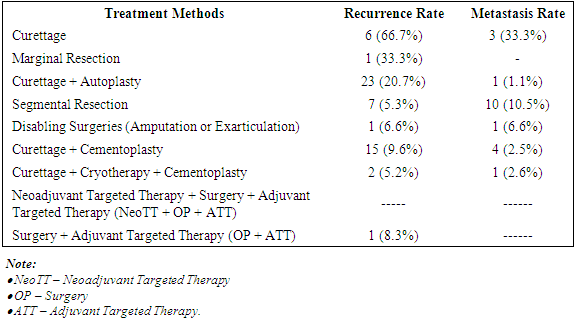 |
| |
|
The frequency of tumor recurrence and metastasis after various treatment methods is presented in Table 4. Let's consider each of the methods and their results in more detail: after surgery, tumor excochleation, the recurrence rate was 66.7%, and metastases were 33.3%. This indicates a high frequency of relapses and metastases compared to other visible treatments for GKD.When performing intraosseous marginal resection, the recurrence rate is 33.3%. Moderate risk of recurrence and absence of metastases make this method more successful than excochleation.After excochlear surgery and autoplasty, the frequency of relapses decreases to 20.7%, and metastases to 1.1% of cases. Adding autoplasty reduces the risk of recurrence and metastasis, making this method more effective.Following segmental bone resection, the recurrence rate was 5.3%, and metastases were 10.5%. Although the risk of recurrence is minimal, metastases are more common than some other methods.The frequency of relapses during paresis operations (amputation or exarticulation) was 6.6%, and metastases were 6.6%. This method is characterized by a low risk of both relapses and metastases, but its application is limited by severe functional and social consequences.In recent years, orthopedic practice has been focusing on the use of minimally invasive methods for treating giant bone tumors using targeted drugs. In this case, excochleation is performed in combination with neoadjuvant and adjuvant targeted therapy with the drug Denosumab (Prolia, Exjiva).In our observations, excochleation with cement plasty was performed on 156 patients. Meanwhile, the frequency of relapses was 9.6%, and metastases were 2.5%. Adding cementoplasty reduces the risk of relapses compared to simple excochleation, and the frequency of metastases also decreases compared to pure excochleation.Minimally invasive surgery - excochleation and cryoplasty with cement plasty - reduces tumor recurrence to 5.2%, and metastases to 2.6%. The comprehensive use of these methods indicates a low frequency of relapses and metastases, making it a promising treatment option.The use of the target drug Denosumab in the treatment of giant bone tumors in the mode: neoadjuvant targeted therapy + surgery + adjuvant targeted therapy made it possible to completely eliminate relapses and metastases.When evaluating the effectiveness of surgery + adjuvant targeted therapy, it was established that the frequency of relapses significantly increased despite the use of Denosumab in an adjuvant mode - 8.3%, and metastases were absent. Targeted therapy surgery shows a relatively low frequency of relapses, with no data on metastases.Methods that include additional procedures (autoplasty, cementoplasty, cryotherapy), as a rule, show a lower frequency of relapses and metastases. Simple excochleation and marginal resection are the least effective in terms of recurrence and metastasis frequency.Traumatic operations and complex treatment methods (for example, targeted therapy) yield good results in reducing the risk, although data on some methods are not fully presented. These data contribute to the evaluation of the effectiveness of various treatment methods and their impact on the long-term outcome of tumor treatment.
3. Conclusions
Thus, the analysis of the evolution of surgical treatment for giant cell tumors of bone (GCT) has shown that the use of denosumab in neoadjuvant and adjuvant regimens provides a good clinical and morphological effect, with no tumor recurrence or metastasis observed. This treatment regimen can be widely implemented in clinical practice for the combined treatment of GCT, even in cases of large bone lesions. The treatment method allows for the preservation of limb function and prevents the need for segmental bone resections with autografting or large joint endoprosthesis.Statistical analysis has demonstrated that when determining the extent of surgical intervention, it is necessary to consider the informativeness and relative importance of clinical, radiological, and morphological features as a whole. This approach contributes to the prevention of tumor recurrence and metastasis, consequently improving long-term outcomes. The criteria we propose for predicting the development of tumor recurrence and metastases allow for the correct selection of surgical intervention method in each specific case.
References
| [1] | Saparbayev A. I., Saparbayev A. I., Gafur-Akhunov M. A., Nabieva D. U., & Yigitaliev A. B. (2024). Modern aspects of diagnostics and treatment of metastatic lung lesions in bone sarcomas. Research journal on traumatology and disability, 3(10), 130–146. |
| [2] | Amanov V. R. Development of new approaches in preservative-restorative treatment of tumors and tumor-like lesions of long tubular bones. Abstract of PhD thesis. – Tashkent, 2004. – 18 p. |
| [3] | Tozhiboev A. A., Gafur-Akhunov M. A., Polatova D. Sh., Abdikarimov H. G. Methods of application of minimally invasive methods of treatment of giant cell tumor of tubular bones (Methodological recommendations). Tashkent, 2018. |
| [4] | Tozhiboev A. A. Improving minimally invasive methods of complex treatment of giant cell tumors of tubular bones. Abstract of a PhD diss. in medical sciences. - Tashkent, 2019. - 28 p. |
| [5] | Muminov Sh. M. Evaluation of the effectiveness and choice of treatment method for giant cell tumors of tubular bones. Abstract of a PhD diss. - Tashkent, 2003. - 23 p. |
| [6] | Aliev M. D. Malignant bone tumors. Bone, soft tissue sarcomas and skin tumors 2010; 2: 3-8. |
| [7] | Aliev M. D., Sushentsov E. A. Modern oncoorthopedics. Bone, soft tissue sarcomas and skin tumors 2012; 4: 3-10. |
| [8] | Bludov A.B., Nered A.S., Zamogilnaya Ya.A., Kochergina N.V. Giant cell tumor of bone // Sarcomas of bones, soft tissues and skin tumors. - 2014. - Nº 1. - P. 16-34. |
| [9] | Gafur-Akhunov M.A, Nabieva D.U., Kasimov U.K., Tursinov I.T., & Sharipov M.M. (2024). Clinical and Morphological Characteristics of the Efficiency of Targeted Therapy in Patients with Giant Cell Tumor of Tubular Bones. Research Journal of Trauma and Disability Studies, 3(10), 121–129. |
| [10] | Valiev A.K., Teplyakov V.V., Musaev E.R., Rogozhin D.V., Sushentsov E.A., Machak G.N. et al. Practical recommendations for the treatment of primary malignant bone tumors. malignant tumors: Practical recommendations RUSSCO #3s2, 2022 (volume 12). 307-329. |
| [11] | Gershtein E.S., Timofeev Yu.S., Zuev A.A. et al. The RANK/RANKL/OPG ligand-receptor system and its role in primary bone neoplasms (literature analysis and our own results) // Advances in Molecular Oncology. - 2015. - No. 2. - P. 51-59. |
| [12] | Grigorovsky V.V. Giant cell tumor of bone: morphogenesis, clinical and morphological features, differential diagnostics, approaches to treatment // Oncology (Kyiv). - 2012. - Vol. 14, No. 1. - P. 64-76. |
| [13] | Grigorovsky V.V., Kris-Pugach A.P., Luchko R.V. et al. Giant cell proliferative bone lesions // Orthopedics and traumatology. - 2001. - No. 1. - P. 120-127. |
| [14] | Demichev N.P., Ivanov V.N. Differential diagnostics of giant cell tumors of bone // Orthopedics and traumatology. - 1991. - No. 6. - P. 51-58. |
| [15] | Zaitseva M.Yu., Zasulsky F.Yu. Morphological features of variants of the structure of giant cell tumor of bones // Traumatology and orthopedics of Russia. - 2010. - Vol. 16, No. 1. - P. 139-146. |
| [16] | Krzhivitsky P.I. Clinical and radiation diagnostics of bone sarcomas // Practical oncology. - 2010. - No. 1, - P. 11-18. |
| [17] | Practical recommendations of the Russian Society of Clinical Oncology. Malignant tumors. Vol. 9 No. 3s2. 2019. p-265. |
| [18] | Becker W., Dohle J., Bernd L. et al. Local recurrence of giant cell tumour of bone after intralesional treatment with and without adjuvant therapy // J. Bone Joint Surg. - 2008. - Vol. 90, Ng 5. - P. 1060-1067. |
| [19] | Bertoni F., Bacchini P., Staals E. L. Malignancy in giant cell tumour // Skelet Radiol. - 2003. - Vol. 32, Nº 3. - P. 143-146. |
| [20] | Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of Denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 2013; 14: 901-8. |
| [21] | Dickson B.C., Li S.Q., Wunder J. et al. Giant cell tumor of bone express p63 // Mod Pathol. - 2008. - Vol. 21, Nº 4. - P. 369-375. |
| [22] | Errani C., Tsukamoto S., Leone G. et al. Denosumab May Increase the Risk of Local Recurrence in Patients with Giant-Cell Tumor of Bone Treated with Curettage // J. Bone Joint Surg. Am. - 2018. - Vol. 100. - P. 496. |
| [23] | Gortzak Y., Kandel R., Deheshi B. et al. The efficacy of chemical adjuvants on giant-cell tumour of bone // J. Bone Surg. Brit. - 2010. - Vol. 92. - P. 1475. |
| [24] | Lim C.Y., Liu X., He F. et al. Retrospective cohort study of 68 sacral giant cell tumours treated with nerve-sparing surgery and evaluation on therapeutic benefits of denosumab therapy // Bone Joint J. - 2020. - Vol 102, Nº 2. - P. 177-185. |
| [25] | Martin-Broto J, Cleeland CS, Glare PA, et al. Effects of Denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol 2014; 53: 1173-9. |
| [26] | Palmerini E., Chawla N.S., Ferrari S. et al. Denosumab in advanced unresectable giant-cell tumor of bone (GCTB): For how long // Eur. J. Cancer. - 2017. - Vol. 76. - P. 118-124. |



















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


