-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(11): 2836-2840
doi:10.5923/j.ajmms.20241411.35
Received: Oct. 25, 2024; Accepted: Nov. 10, 2024; Published: Nov. 13, 2024

Significance of Salmonellosis Among Registered Acute Intestinal Infections in the Samarkand Region
Bakhriyeva Z. D.1, Mirzadjanova D.2
1Chair of Infectious Diseases, Samarkand State Medical University, Samarkand, Uzbekistan
2Republican Specialized Scientific and Practical Medical Center of Epidemiology, Microbiology, Infectious and Parasitic Diseases, Tashkent, Uzbekistan
Correspondence to: Bakhriyeva Z. D., Chair of Infectious Diseases, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this study was to evaluate the prevalence of salmonellosis within the total registered cases of acute intestinal infections (AIIs) in the Samarkand region. To achieve this, official data on AII incidence over the studied period were collected from the Center for Sanitary and Epidemiological Welfare and Public Health of the Republic of Uzbekistan, enabling a comprehensive retrospective analysis. The analysis revealed an upward trend in the incidence of acute intestinal infections in the Samarkand region, reflecting similar patterns observed in other regions across Uzbekistan. However, bacteriological methods confirmed an etiological factor in only one out of every seven registered AII cases, underscoring a diagnostic gap in routine pathogen identification. Among AIIs with a confirmed etiology, salmonellosis accounted for an average of 3.6%, suggesting it plays a relatively modest but significant role in the epidemiology of intestinal infections in this region. These findings highlight the need for enhanced diagnostic capabilities and pathogen-specific surveillance to accurately capture the burden and dynamics of salmonellosis within broader AII patterns in Uzbekistan.
Keywords: Salmonellosis, Samarkand region, Incidence rate, Children
Cite this paper: Bakhriyeva Z. D., Mirzadjanova D., Significance of Salmonellosis Among Registered Acute Intestinal Infections in the Samarkand Region, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2836-2840. doi: 10.5923/j.ajmms.20241411.35.
1. Introduction
- Diarrheal diseases remain a major global public health issue, with an estimated 90 million cases of gastroenteritis and approximately 155,000 related deaths each year, as reported by the World Health Organization (WHO) [1]. Among the causative agents, Salmonella is responsible for nearly one-quarter of diarrheal illnesses worldwide, highlighting its significant burden on healthcare systems and populations [2].Salmonellosis incidence varies widely by region and over time. In 2017, for instance, Poland reported 1,000 cases of Salmonella infection, corresponding to a rate of 26.0 cases per 100,000 people [3]. Similarly, data from 23 European countries in 2019 documented 926 outbreaks of foodborne salmonellosis, leading to 9,169 reported cases, 1,915 hospitalizations, and 7 deaths. Salmonella was implicated in 17.9% (nearly one in six) of these outbreaks, underscoring its substantial impact on public health across Europe [4]. In the United States, two serovars—S. Typhimurium and S. Enteritidis—account for 41.5% of all Salmonella outbreaks, emphasizing their relevance in the epidemiology of salmonellosis [5]. The Russian Federation also reported similar trends between 2015 and 2020, with S. Enteritidis (64.7%), S. Typhimurium (4.8%), and S. Infantis (3.2%) being the most dominant serotypes. S. Infantis was predominantly associated with chicken and turkey, while S. Kentucky and S. Typhimurium were linked to environmental sources and pork products [6].Studies in Kazakhstan, such as those by Kozhakhmetova and colleagues (2019), revealed a similar public health challenge. In Almaty, 269 cases of salmonellosis were reported in 2013, equating to an incidence rate of 17.52 per 100,000 people—a 25.9% reduction from the previous year. Notably, 60 cases were among children under 14, with an incidence rate of 16.17 per 100,000, 44.9 cases lower than in 2012, indicating the substantial risk salmonellosis poses to both adults (77.7% of cases) and children [7,8].In Uzbekistan, research by Almatov B.I. et al. (2018) examined the prevalence of non-typhoid salmonellosis from 2008 to 2017, noting a gradual increase in S. Enteritidis since 2012. By the end of the study, S. Enteritidis nearly matched S. Typhimurium (42.0% and 39.5%, respectively) in prevalence. This shift was particularly evident in Tashkent, where S. Enteritidis rose from 18% to 39% by 2012, ultimately becoming the dominant serovar by 2017, identified three times more frequently than S. Typhimurium (60.5% vs. 19.2%) in patients with acute intestinal infections [9,10].The variability in salmonellosis incidence, strain dominance, and transmission pathways appears to be influenced by a region’s social and environmental factors, as well as local dietary habits and cultural practices [9]. In Uzbekistan, salmonellosis prevalence has been explored at the national level and in Tashkent; however, there is limited data on the incidence, leading strains, and transmission routes specific to the Samarkand region and its districts [11]. Given these gaps, the current study aimed to determine the proportion of salmonellosis among registered cases of acute intestinal infections in the Samarkand region, contributing to a better understanding of salmonellosis epidemiology in this region.
2. Materials and Methods
- This study utilized a retrospective analysis of acute intestinal infection (AII) cases recorded in the Samarkand region from 1991 to 2023. Data were collected from the official records maintained by the Center for Sanitary and Epidemiological Welfare and Public Health of the Republic of Uzbekistan. The analysis included all recorded cases of AII within this timeframe, focusing on trends in incidence, confirmation rates of bacterial etiology, and the proportion of cases identified as salmonellosis.Data Collection and Study Population: The study population consisted of all registered cases of AII in the Samarkand region between 1991 and 2023. Data were extracted on incidence rates per 100,000 population for each year, as well as information on bacteriological testing and confirmed etiologies. Additionally, data on salmonellosis cases within the subset of bacteriologically confirmed AIIs were collected to determine the relative burden of salmonellosis in the region.Bacteriological Testing: Cases underwent bacteriological examination to confirm etiological agents. Testing methods included stool culture analysis, where samples were screened for common bacterial pathogens such as Salmonella spp., Shigella spp., and Campylobacter spp., following standard diagnostic protocols established by the Center for Sanitary and Epidemiological Welfare. Positive cases of salmonellosis were further categorized by serovars, including S. Enteritidis, S. Typhimurium, and other regional strains where identified.Data Analysis: A statistical analysis of incidence trends was conducted to examine AII cases over time. Annual incidence rates were calculated per 100,000 population, allowing for trend comparison over the 32-year period. The proportion of cases with a confirmed bacterial etiology was calculated for each year, and these proportions were used to evaluate trends in diagnostic confirmation rates. The data were also stratified by year to analyze the proportion of salmonellosis within confirmed cases of AII.For statistical reliability, comparisons were drawn between the Samarkand region’s AII trends and national data from the Republic of Uzbekistan, focusing on periods of notable incidence fluctuation, such as the COVID-19 pandemic. Incidence trends were analyzed to identify periods of increase, decrease, and stability, including wave-like patterns observed in specific periods. Comparative analysis assessed differences between national and regional trends to evaluate regional variability in AII incidence.
3. Results
- This study investigated the incidence of AII in the Samarkand region from 1991 to 2023, with data sourced from the Center for Sanitary and Epidemiological Welfare and Public Health of Uzbekistan. The incidence rate of AII in Samarkand began at 535.8 cases per 100,000 population in 1991, declining steadily to 84.5 cases per 100,000 by 2003. Between 2003 and 2019, the incidence showed a wave-like pattern, consistently remaining below 100 cases per 100,000, but above 80 cases. During the COVID-19 pandemic in 2020, a sharp drop from 231.1 to 63.8 cases per 100,000 was observed, followed by a rebound to 123.7 cases per 100,000 in 2021. A further increase led to an unprecedented high of 203.5 cases per 100,000 by 2023.When examining confirmed versus unconfirmed cases, we observed that only a minority had an etiological factor confirmed via bacteriological testing. In 1991, bacteriological testing identified an etiological agent in just 1 out of every 5 cases, with the remainder classified as acute diarrhea of unknown etiology. This ratio improved to 1 in 3 by 2003 but later declined, with only 1 in 7 cases confirmed by 2023 (Figure 1).
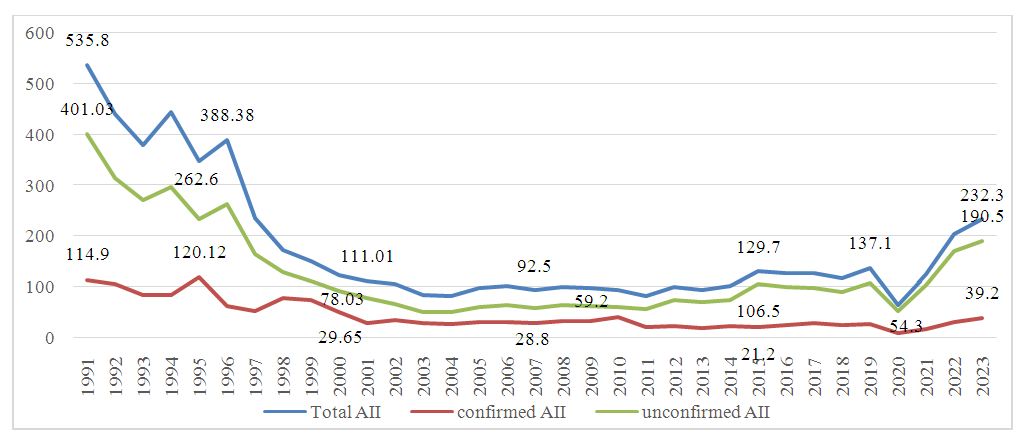 | Figure 1. Analysis of Registered AII Cases in the Samarkand Region (Incidence Rate) |
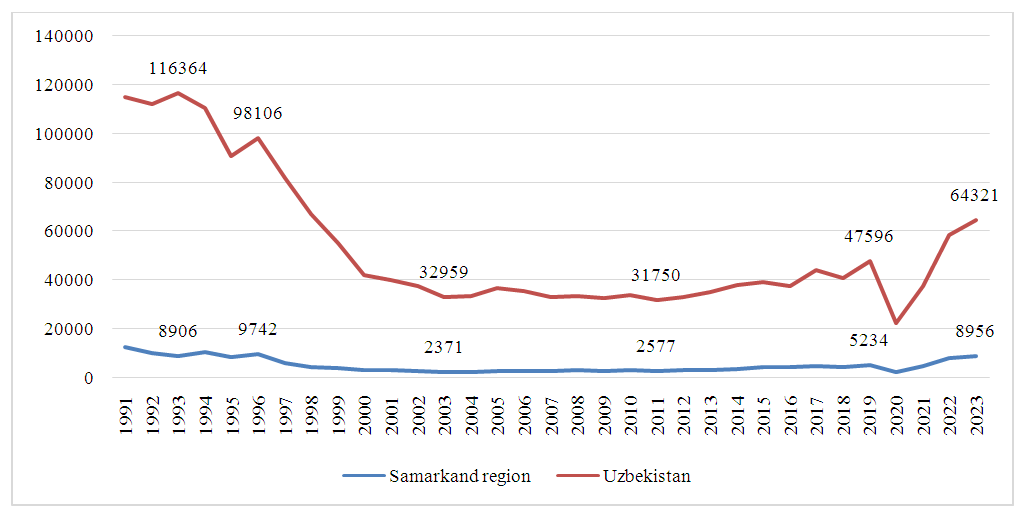 | Figure 2. Comparative Analysis of AII Incidence in the Samarkand Region and National Trends in Uzbekistan |
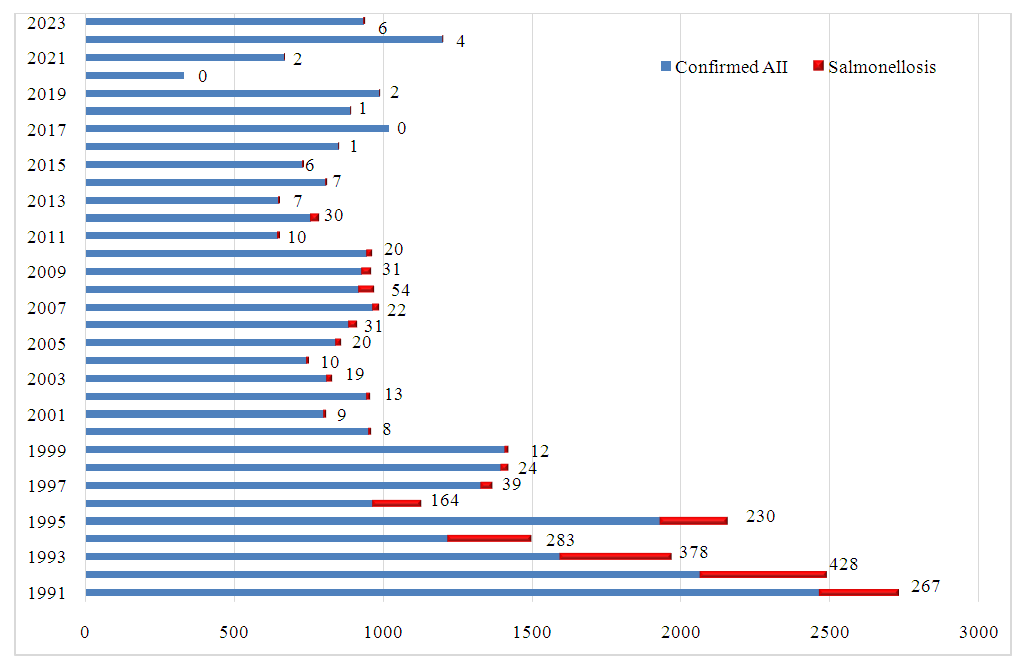 | Figure 3. Proportion of Confirmed Salmonellosis Cases Among Confirmed AII Cases in the Samarkand Region (Absolute Values) |
4. Conclusions
- The incidence of AII in the Samarkand region shows an increasing trend, consistent with national patterns in Uzbekistan. Only 1 in 7 registered AII cases has a confirmed bacterial etiology, with salmonellosis accounting for 3.6% of these confirmed cases. This limited etiological confirmation underscores a diagnostic gap that challenges accurate epidemiological assessment and the implementation of targeted interventions for salmonellosis and other AIIs. Given the rising incidence and the potential for region-specific factors affecting disease spread, enhanced diagnostic capacity, including PCR and expanded bacteriological testing, is recommended to improve AII management in the Samarkand region.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML