-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(11): 2697-2700
doi:10.5923/j.ajmms.20241411.03
Received: Oct. 11, 2024; Accepted: Nov. 3, 2024; Published: Nov. 7, 2024

Ischemic Stroke in Young Adults Due to Protein C, Protein S, and Antithrombin III Deficiency
Dilshod Tolibov1, Nargiza Qarshiboyeva2
1DSc, Associate Professor, Tashkent Medical Academy, Tashkent, Uzbekistan
2Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Deficiency of the coagulation system inhibitors protein C, protein S, and antithrombin III increases the risk of venous thrombosis. We describe 10 young patients with cerebral arterial thrombosis caused by deficiency of these factors. 60 patients under 50 years of age were diagnosed by computed tomography or magnetic resonance imaging and hospitalized for acute ischemic stroke. Cerebral angiography was performed in 54 patients. All patients were screened for hematological and coagulation factors and antibodies. Among general cases, Holter monitoring was performed in 13 patients, and echocardiography was performed in 20 patients. Quantitative analysis of protein C, protein S (by Laurel rocket immunoelectrophoresis) and antithrombin III (by radial immunodiffusion) was performed in all patients and six patients' relatives after stroke for 3 months. 10 cases (17%) were caused by coagulation inhibitor deficiency: three males had protein C deficiency, two females had protein C deficiency, and five (three males and two females) had antithrombin III deficiency. In these 10 patients, cerebral ischemia involved the carotid region. No one had a history of thromboembolic disease. Eight patients showed complete recovery. Impairment was predicted in patients with one acquired protein C deficiency and two with antithrombin III deficiency; the rest were considered heterozygous. Changes in cerebral blood vessels may be primarily related to the lack of these natural anticoagulant substances. The most damage was observed in young patients. Information about these new coagulation defects may lead to improved prevention and treatment of neurological disease.
Keywords: Blood coagulation system, Brain ischemia, Youth, Antithrombin, Mmunoelectrophoresis
Cite this paper: Dilshod Tolibov, Nargiza Qarshiboyeva, Ischemic Stroke in Young Adults Due to Protein C, Protein S, and Antithrombin III Deficiency, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2697-2700. doi: 10.5923/j.ajmms.20241411.03.
1. Introduction
- About 4% of young people with cerebral ischemia, the main cause of thrombosis is hematological disorders [1] [2]. This figure probably underestimates the role of hemostatic disorders as a cause of cerebrovascular disease. Atherosclerosis is a rare cause of cerebral ischemia in young people [3]. Cardioembolism It is usually the main cause of ischemic stroke among youth [5], [4]. Even after comprehensive investigations, the etiology of cerebral infarction in a significant number of young patients has not been determined [6], [7].Acute ischemic stroke usually has no abnormalities in routine hematologic or coagulation tests [8]. Some authors have reported that hemostatic disturbances in stroke occurring in the young age group include abnormalities of platelet function, inhibition of the coagulation system, or fibrinolysis. Coagulation inhibitory proteins (CIPs) include antithrombin III (ATIII), protein C (PC), and protein S (PS). Currently, the natural anticoagulant pathway in the regulation of thrombosis is better evaluated and understood. Although CIP deficiency usually causes venous thrombosis, CIP inhibits blood coagulation in several ways. ATIII binds to heparin on the surface of endothelial cells, increasing its ability to inhibit thrombin and other activated blood clotting factors. PC is an anticoagulant protease that inactivates thrombin and activated factors V and VIII by reacting thrombin with thrombomodulin on the surface of endothelial cells, and also stimulates fibrinolysis. PS is freely available in the plasma and binds to the inhibitory proteins of the complement system [9], [10]. Only free-form activated factors V and VIII accelerate PC inhibition. Several manuscripts have reported that PC or PS deficiency leads to ischemic stroke.In this article, we describe 10 patients with acute ischemic stroke secondary to CIP deficiency. ATIII deficiency was found in five patients, PC deficiency in three patients, and PS deficiency in two.
2. Method
- From September 2022 to August 2023, 60 patients under the age of 50 (37 men, 23 women) were admitted to the multidisciplinary clinic of the Tashkent Medical Academy within 48 hours after the first ischemic stroke. Patients with a diagnosis of complicated migraine, transient ischemic attacks, intracerebral or subarachnoid hemorrhage were excluded. The diagnosis of all patients was confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). Angiography of cerebral vessels was performed in 54 patients.A clinical profile of hypertension, heart disease, diabetes, migraine, vascular disease, oral contraceptive use was obtained in each patient. It was also noted that acute stroke is associated with pregnancy, alcohol consumption, or acquired viral infection. None of the patients received anticoagulant therapy before or during hospitalization. In all cases, treatment aimed at normalizing blood pressure, mannitol or glycerin osmotic therapy, and use of antiplatelet drugs (ticlopidine or aspirin) in the absence of contraindications were carried out.Electrocardiogram and chest x-ray were performed in all patients. M-mode and two-dimensional echocardiography were performed in 20 patients, 24-hour Holter monitoring in 13 patients, and carotid arteries and transcranial Doppler, color Doppler in 15 patients. Routine hematological profiles including blood analysis parameters, prothrombin time, partial thromboplastin time, fibrinogen, erythrocyte sedimentation rate, biochemical blood analysis (glucose, blood urea nitrogen, creatinine, albumin, bilirubin and cholesterol), checked.CIP was determined within 48 hours of symptom onset and prior to any treatment. Venous blood samples were collected from the antecubital vein of the non-paretic arm using a clean venipuncture. The sample was centrifuged at 1600-200Og for 10 minutes and the plasma was stored at -70°C until analysis. For quantitative study of PC antigen and free PS, (after isolation and precipitation of polyethylene glycol-bound PS) was examined immunologically. The results obtained for PC antigen and free PS antigen are expressed as relative percentages compared to the normal plasma standard. The expected normal range for PC antigen was 65–145%, and this was the coefficient of variation (CV) for the assay. for free PS antigen, the normal range was 60–150%, and the CV was 5.2%. Quantitative analysis of ATIII is performed by adding goat anti-human ATIII (Helena) to agarose using radial immunodiffusion. Results are expressed in milligrams per deciliter, the normal range obtained in our laboratory is 9-14 mg/dL. The obtained CV was 4.1%.
3. Results
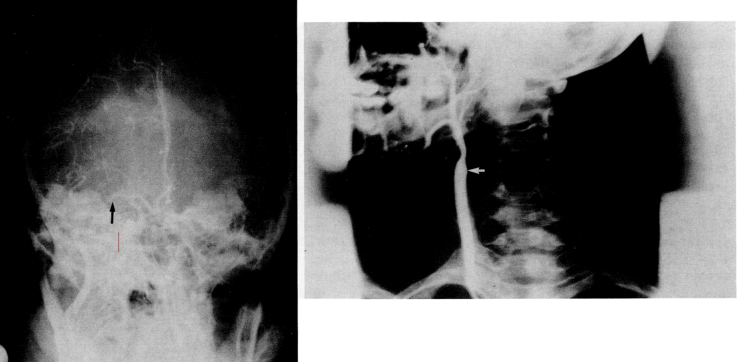
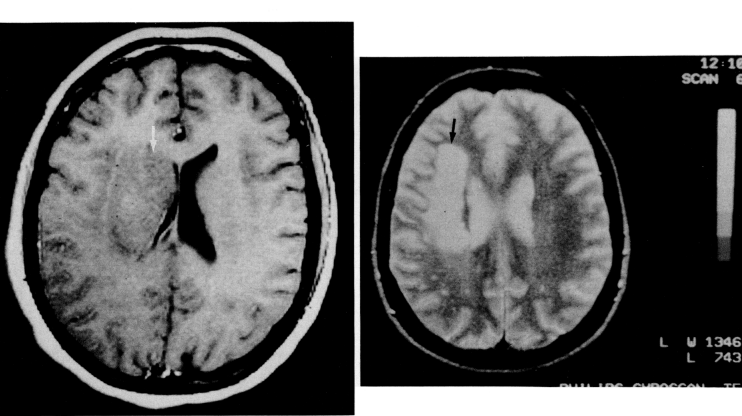
- In one third of 60 patients, the possible etiology of cerebral infarction was not determined. Four patients died within 3 months of cerebrovascular disease, two had systemic medical complications, and two had cardiac or vascular disease (myocardial infarction and Takayasu's disease). In 55 patients (92%), it was observed in the carotid region and in five patients in the brainstem region (two lateral medullary stroke, two lateral pontine stroke, and one mesencephalic region stroke). Stroke was demonstrated by CT in 52 patients and by MRI in the remaining eight.Acute ischemic stroke in 10 patients (17%) was associated with hematological disease. Six of the patients in this group were male and four were female, with a mean age range of 31.4 (24-38). There is no significant difference in mean age between women and men. Table 1 shows the 10 patients and their clinical presentation at admission. Nine patients underwent cerebral angiography, and eight patients showed either an occluded vessel or partial intravascular filling.
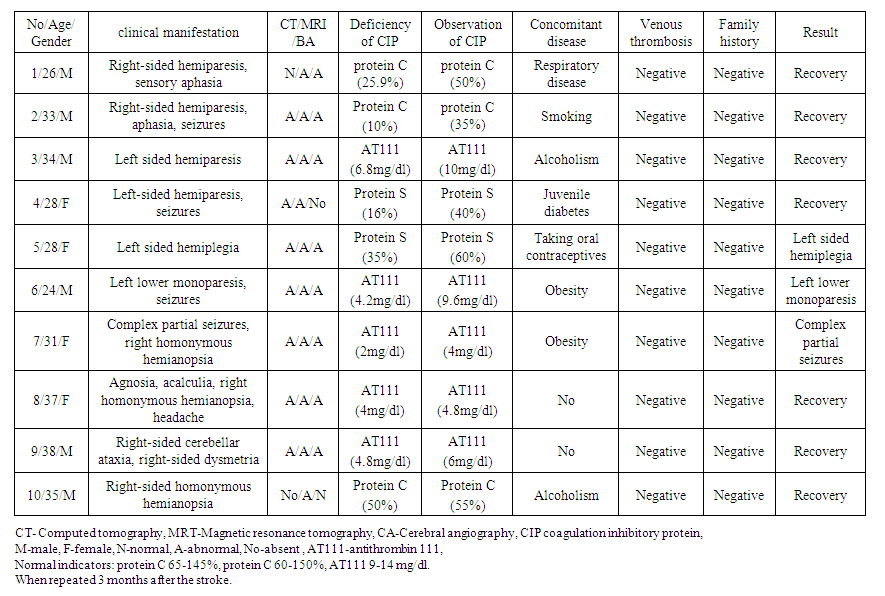 | Table 1. 10 patients at the time of admission and their clinical manifestations |
4. Discussion
- The etiology of stroke in young people is unknown in most cases. In this age group, approximately 4% of stroke etiology is hematological diseases. Currently, increased prothrombin level in laboratory screening of stroke patients increases the rate of stroke.Routine hematological or coagulation tests are usually normal in acute ischemic stroke. In 21 patients (35%), the etiology of stroke remained undetermined. 10 (17%) patients with ischemic stroke had a hematological disease. In contrast to other undocumented reports, we found deficits in CIP in stroke patients, with PC deficiency in three males, PS deficiency in two females, and ATIII deficiency in two females and three males.PC is a vitamin K-dependent zymogen of plasma serine protease. After activation in vivo by the reaction of thrombin with endothelial thrombomodulin, it inhibits blood coagulation under the influence of activating factors Va and Vlla. Also, the fibrinolytic system produces tissue plasminogen activator, neutralizes the activity of plasminogen activator inhibitors and ensures that they work in the same balance.Hematological diseases are one of the causes of ischemic stroke that often occurs in young people. Compared to the elderly, atherosclerotic mechanisms are less common in patients of this age group. 3 If a hematological anomaly is detected after a cerebrovascular disease, it should not be associated with a previous stroke. In order to be associated with a previous stroke, the abnormality must persist in the following months or be found in family members. In our patients, the levels of PC (three patients), PS (one patient) and ATIII (three patients) remained low at 3 months after stroke, supporting the hypothesis that hematological deficits are the cause rather than the consequence of stroke. For a more complete understanding of blood clotting mechanisms and blood clotting, a thorough laboratory screening is performed. This makes it possible to learn about new pathophysiological mechanisms, to improve methods of prevention and treatment of neurological diseases.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML