-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(11): 2693-2696
doi:10.5923/j.ajmms.20241411.02
Received: Oct. 12, 2024; Accepted: Oct. 28, 2024; Published: Nov. 7, 2024

Ulinastatin in Complex Therapy of Patients with Acute Pancreatitis
Murotov Temur Malik1, Irnazarov Shahzod2, Igamkulov Behzod2, Davronov Sarvarjon2, Nasritdinov Biloliddin2, Juraqulov Abbosjon3
1Phd, Associate Professor, Department of Anesthesiology and Resuscitation of the Tashkent Medical Academy, Tashkent, Uzbekistan
2Master, Department of Anesthesiology and Resuscitation of the Tashkent Medical Academy, Tashkent, Uzbekistan
3Assistent, Department of Professional Sciences, Faculty of Medicine, Angren University, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

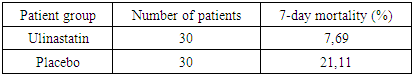
The aim of this study was to evaluate the effectiveness of ulinastatin in the comprehensive treatment of patients with severe acute pancreatitis (SAP) [1]. The study was conducted in the intensive care unit with 60 patients who were randomized into two groups: one received ulinastatin (100,000 IU three times a day), and the other received a placebo. The primary endpoints included 7-day mortality, levels of inflammatory markers (TNF-α, IL-6), coagulation parameters, and organ function. The results showed a significant reduction in mortality and improvement in clinical outcomes in the ulinastatin group compared to the placebo group. Additionally, there was a decrease in inflammatory cytokines and improvements in liver and kidney function. The use of ulinastatin in the comprehensive therapy of SAP reduces mortality and improves clinical outcomes without significant side effects [5].
Keywords: Acute pancreatitis, Ulinastatin, Inflammation, Coagulation, Multiple organ failure, Serine proteases, Clinical efficacy
Cite this paper: Murotov Temur Malik, Irnazarov Shahzod, Igamkulov Behzod, Davronov Sarvarjon, Nasritdinov Biloliddin, Juraqulov Abbosjon, Ulinastatin in Complex Therapy of Patients with Acute Pancreatitis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 11, 2024, pp. 2693-2696. doi: 10.5923/j.ajmms.20241411.02.
Article Outline
1. Introduction
- Severe acute pancreatitis (SAP) represents one of the most severe diseases of the pancreas, characterized by deficits and risk of multi-organ failure. In this case (ACE), the mortality rate reaches 25-40%, especially in the setting of systemic inflammatory response and necrotic tissue damage. Over the last decade, there has been an intensive increase in the incidence of acute pancreatitis among young adults and adolescents. The peculiarity of the course of pancreatitis in young people is the high risk of complications, with a mortality rate of up to 5.5 per cent [2].Over the past few years, Uzbekistan has seen an increase in the incidence of acute pancreatitis. The incidence of acute pancreatitis has been increasing in Uzbekistan over the previous years. In the composition of emergency surgical pathology this disease takes the 3rd place, yielding to acute appendicitis and gallbladder pathology, and makes up to 10-16%. According to the literature, about 140 factors that can provoke the development of acute pancreatitis have been found. Severe destructive forms of acute pancreatitis are registered in 15-30% of patients. Up to 80% of causes of death of patients with acute destructive pancreatitis are caused by infectious complications of abdominal cavity and retroperitoneal space, systemic infectious complications.In this regard, the search for effective treatment methods is acute. Ulinastatin, a serine protease inhibitor, has demonstrated efficacy in the care of SST due to its anti-inflammatory and immunomodulatory properties [6,8].Purpose of the studyThe aim of the present study was to evaluate the efficacy of ulinastatin in the complex therapy of patients with severe acute pancreatitis (SAP).Objectives of the study:1. To determine the optimal protease inhibitor therapy for the organism and to evaluate the effect of ulinastatin on 7-day mortality in patients with SARS.2. To investigate changes in the levels of inflammatory markers (TNF-α, IL-6) before and after treatment.3. To study the effect of ulinastatin on coagulation indices, liver and kidney function.4. To determine the safety of ulinastatin in patients with severe acute pancreatitis.
2. Materials and Methods of Research
- The study was conducted in the intensive care unit (ICU) with the involvement of patients diagnosed with severe acute pancreatitis (SAP). The main aim of the study was to evaluate the efficacy of ulinastatin in the complex therapy of SAR, its effect on mortality, systemic recovery, coagulation function, as well as liver and renal function.Study designThis was a randomised blinded controlled trial involving two groups of patients: one group received ulinastatin (300,000 IU daily) and the other group received placebo. The drugs were administered intravenously for 7 days. All other treatments, including nutritional support and symptomatic therapy, were the same in both groups.Inclusion CriteriaPatients aged 25 to 70 years fulfilling the criteria were included in the study:- Diagnosis of severe acute pancreatitis according to the revised Atlanta classification.- Admission to the ICU within 48 hours of symptom onset.- Presence of evidence of organ dysfunction such as coagulation, renal or hepatic dysfunction confirmed by laboratory tests.Exclusion criteriaPatients were excluded from the study if they had:- Allergy to ulinastatin.- Malignant neoplasms.- Taking immunosuppressants 48 hours prior to enrolment.- Pregnancy.Reason for randomisationRandomisation was performed using computer software to create random sequences, resulting in an even distribution of patients in the treatment and placebo groups in a 1:1 ratio. Both physicians and patients were unaware of which drug was prescribed.Primary endpointsThe primary endpoints were the determination of optimal protease inhibitor therapy for the organism and 7-day mortality associated with the occurrence of severe acute pancreatitis. Additional indices:- Inflammatory cytokine levels (TNF-α, IL-6) before and after treatment.- Coagulation parameters: prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen level.- Liver and renal function measured by the direction of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine and blood urea nitrogen (BUN).- Length of hospital stay and treatment costs.Statistical analysisStatistical analysis was performed using SPSS software version 19.0. Mean values and standard deviations were calculated for the different principles. Between measurements results were performed using t-test for independent samples and χ² test for qualitative data. The level of error was set at p <0.05.Safety of treatmentMultiple complications including granulocytopenia, liver dysfunction, diarrhoea and allergic conditions were closely monitored during the study. Adverse events were recorded every minute during the first 14 days.Thus, the applied methods allow to provide high reliability of results and objective assessment of ulinastatin efficacy in complex therapy of patients with severe acute pancreatitis.Mechanism of action of ulinastatinUlinastatin has a complex action, inhibiting the activity of serine proteases, which leads to the destruction of pancreatic tissues and the development of the inflammatory cascade. It blocks the activation of trypsin, chymotrypsin, thrombin and kallikrein, which reduces the load and slows down the process of tissue self-digestion. Also ulinastatin affects the coagulation system, reducing the risk of coagulopathies, improving microcirculation and preventing the formation of blood clots. A key effect of the drug is to reduce levels of inflammatory cytokines such as interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α), which reduces systemic health and low risk of multi-organ failure [7]. Clinical studies and their resultsNumerous distinctive studies have confirmed the efficacy of ulinastatin in the treatment of patients with acute pancreatitis. One study by Wang S.K [1] et al. demonstrated reduced mortality and improved outcomes in patients receiving ulinastatin compared to a control group receiving placebo.
|
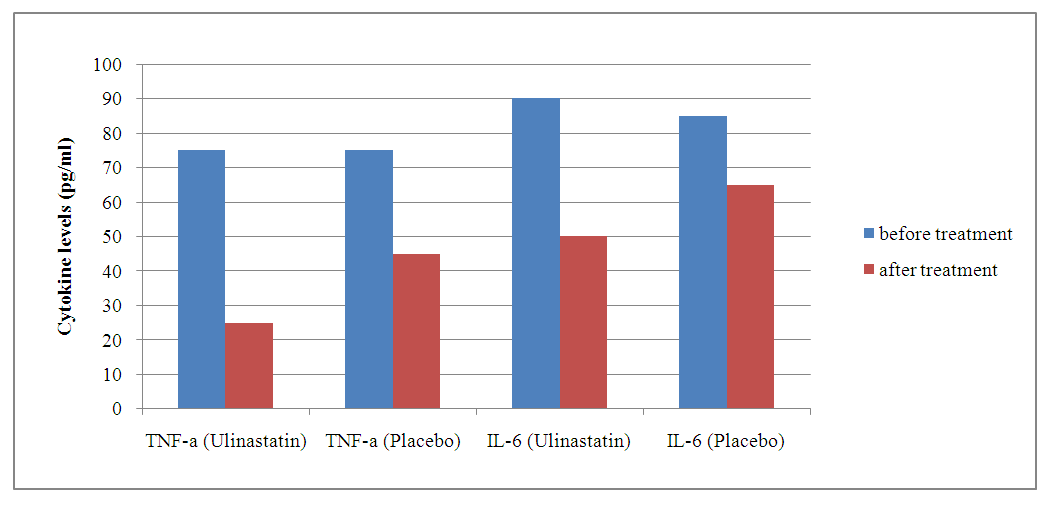 | Graph 1. Dynamics of TNF-α and IL-6 levels before and after treatment |
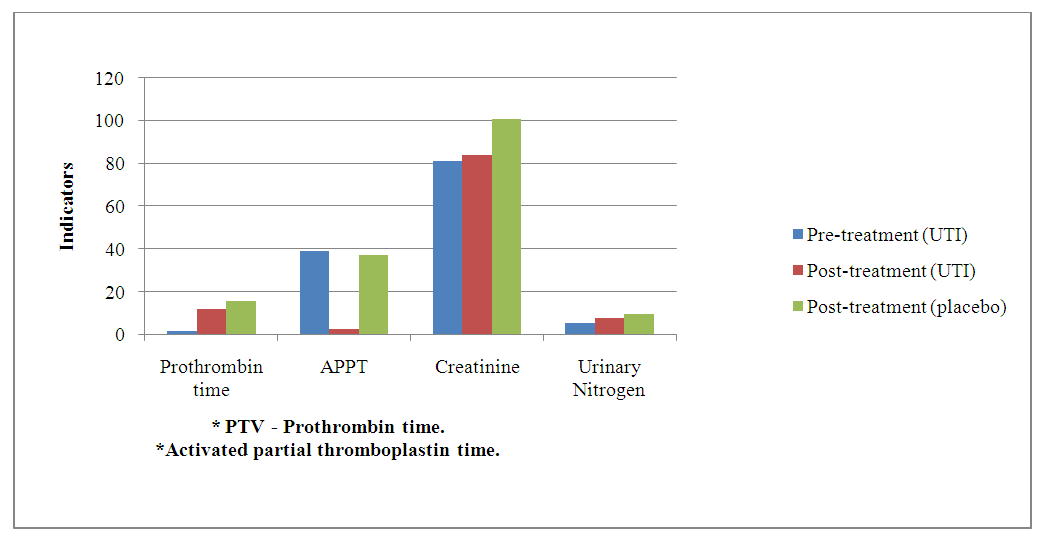 | Grapg 2. Dynamics of internal organ functions before and after treatment |
|
3. Results and Their Discussion
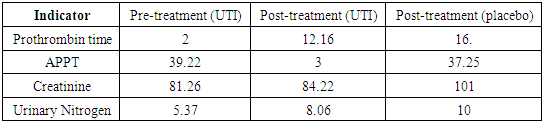
- The results of the study demonstrated significant benefits of ulinastatin compared to placebo in patients with severe acute pancreatitis.1. Reduction in 7-day mortality: The mortality rate was 7.69% in the group receiving ulinastatin, which was significantly lower compared to 21.11% in the placebo group (p = 0.010). These findings are consistent with other studies where ulinastatin reduced mortality and improved clinical outcomes in acute inflammatory diseases [3].2. Reduced levels of inflammatory markers: Patients receiving ulinastatin showed a significant reduction in TNF-α and IL-6 levels compared to controls (p < 0.001). This indicates a pronounced anti-inflammatory effect of the drug, which prevents the development of systemic inflammatory response and multi-organ failure [6].3. Improvement of coagulation parameters: Prothrombin time and APPT decreased significantly after treatment with ulinastatin (p < 0.001), indicating restoration of normal coagulation activity. These results are important to prevent complications associated with coagulation abnormalities in patients with VTE.4. Liver and renal function: Ulinastatin administration improved liver and renal function as reflected by a decrease in creatinine and urea nitrogen levels. Renal indices remained significantly higher in the placebo group, indicating a positive effect of ulinastatin on these organs [5].5. Safety: There were no serious side effects associated with the use of ulinastatin during the study. The most frequent complications were granulocytopenia and liver function abnormalities, but their incidence was slightly higher than in the placebo group and not clinically significant.
4. Conclusions
- The use of ulinastatin in the complex therapy of patients with severe acute pancreatitis effectively reduces mortality, decreases in this level of inflammatory cytokines, improvement of coagulation indices, liver and kidney function. Ulinastatin had a pronounced anti-inflammatory and organoprotective effect, which makes it the main component of therapy of severe acute pancreatitis. The use of ulinastatin also entailed a low level of exposure, which indicates its safety and efficacy of use in clinical practice in patients with severe symptomatology of the disease.To further improve the evidence base and determine optimal doses of ulinastatin, large multicentre studies are needed, as well as long-term studies to assess the long-term effects of the drug [2].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML