Eshankulov Sardor1, Turakulov Rustam2, Bakaev Iskandarbek3, Razzokov Islomjon4
1Head of the Reception Department, National Medical Center, Tashkent, Yashnobod District, Small Ring Road, 151, Pushkin Metro Station, Uzbekistan
2Department of Internal Medicine in Family Medicine No. 2, Tashkent Medical Academy, Farabi street 2, Tashkent, Uzbekistan
3Department of Cardioresuscitation, National Medical Center, Tashkent, Yashnobod District, Small Ring Road, 151, Pushkin metro Station, Uzbekistan
4Department of Rehabilitation, National Medical Center, Tashkent, Yashnobod District, Small Ring Road, 151, Pushkin Metro Station, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
To evaluate the potential role of Helicobacter pylori (H. pylori) infection as an aetiological factor in adult patients suffering from iron-dependent or iron-refractory anemia of unknown cause. METHODS: A prospective evaluation was conducted on consecutive individuals who had H. pylori infection, chronic iron-deficient anemia (IDA) in chronic heart failure. They were all dependent on iron or refractorily iron. The response of H.pylori eradication was evaluated six and twelve months after the follow-up. When the infection was completely eradicated without the need for iron supplements, the H. pylori infection was thought to be the source of the anemia. RESULTS: Of the 89 patients, 88 had no trace of H. pylori. The four eradicating regimens did not work on the non-eradicated patient. Four patients had protocol breaches, making it impossible for them to get treatment.
Keywords:
Chronic Heart Failure (CHF), Iron Deficiency Anemia (IDA), Helicobacter pylori (H. pylori), Gastrointestinal (GI) Tract, Microcytic Anemia, Hypochromic Anemia, Inflammation, Chronic Disease
Cite this paper: Eshankulov Sardor, Turakulov Rustam, Bakaev Iskandarbek, Razzokov Islomjon, Iron Deficiency Anemia Caused By Helicobacter Pylori in Chronic Heart Failure, American Journal of Medicine and Medical Sciences, Vol. 14 No. 10, 2024, pp. 2672-2678. doi: 10.5923/j.ajmms.20241410.47.
1. Introduction
Iron deficiency, with or without anemia, is one of the most prevalent micronutrient deficiencies worldwide, affecting more than a quarter of the population. An estimated 1.14 billion people are affected by anemia, with iron deficiency as an underlying cause in 50% of cases. The highest prevalence is seen in developing countries, and there is a significant disease burden in children under five years of age and in females of reproductive age. In Europe, 27 million individuals are expected to have anemia, including more than 10 million with iron deficiency anemia, and more than 24 million individuals with iron deficiency without anemia [1,2]. This public health concern in developed countries is complemented by high healthcare costs. The economic burden of iron deficiency without anemia has been assessed in Uzbekistan, where healthcare and lost productivity costs reached €3.89 billion (Fig. 1).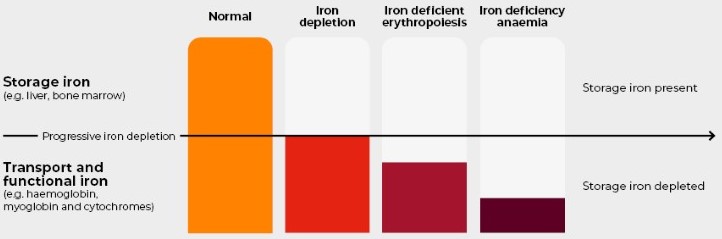 | Figure 1. Iron deficiency without anaemia |
Chronic heart failure (CHF) is a debilitating syndrome with significant morbidity and mortality, largely affecting an older population with multimorbidity and polypharmacy. The Framingham study showed that CHF prevalence increased to 8.4% in persons over 80 years of age. Iron deficiency, with or without anemia, is common in CHF and is associated with poor clinical outcomes. Iron deficiency is thought to be part of the disease process, although it can have multiple secondary factors, including blood loss due to antithrombotic medication, chronic inflammation, renal dysfunction, reduced dietary intake and absorption, and malnutrition. In addition, CHF patients are often subjected to greater blood testing and more invasive procedures, increasing the risk of blood loss.The pathophysiology of iron deficiency in CHF is, to a large extent, multifactorial. Gastrointestinal and endogenous losses of iron can have greater pathological significance in this setting. Impairment of the intestinal epithelial barrier associated with cardiac and renal dysfunction has been documented. Dysfunction of the epithelial intestinal barrier allows translocation of bacterial antigens or toxins into the systemic circulation, leading to a state of chronic low-grade inflammation. The Helicobacter pylori plays a role in inflammation of gastric mucosa and dysregulation of gastric acid secretion, which can enhance the postprandial iron malabsorption of nonionic iron salts. H. pylori infection is an established cause of iron deficiency anemia in developing countries, although knowledge of its etiological role in iron deficiency without anemia is limited. H. pylori and iron deficiency without anemia should be considered as comorbidity in CHF (Fig. 2) [3,4,5]. 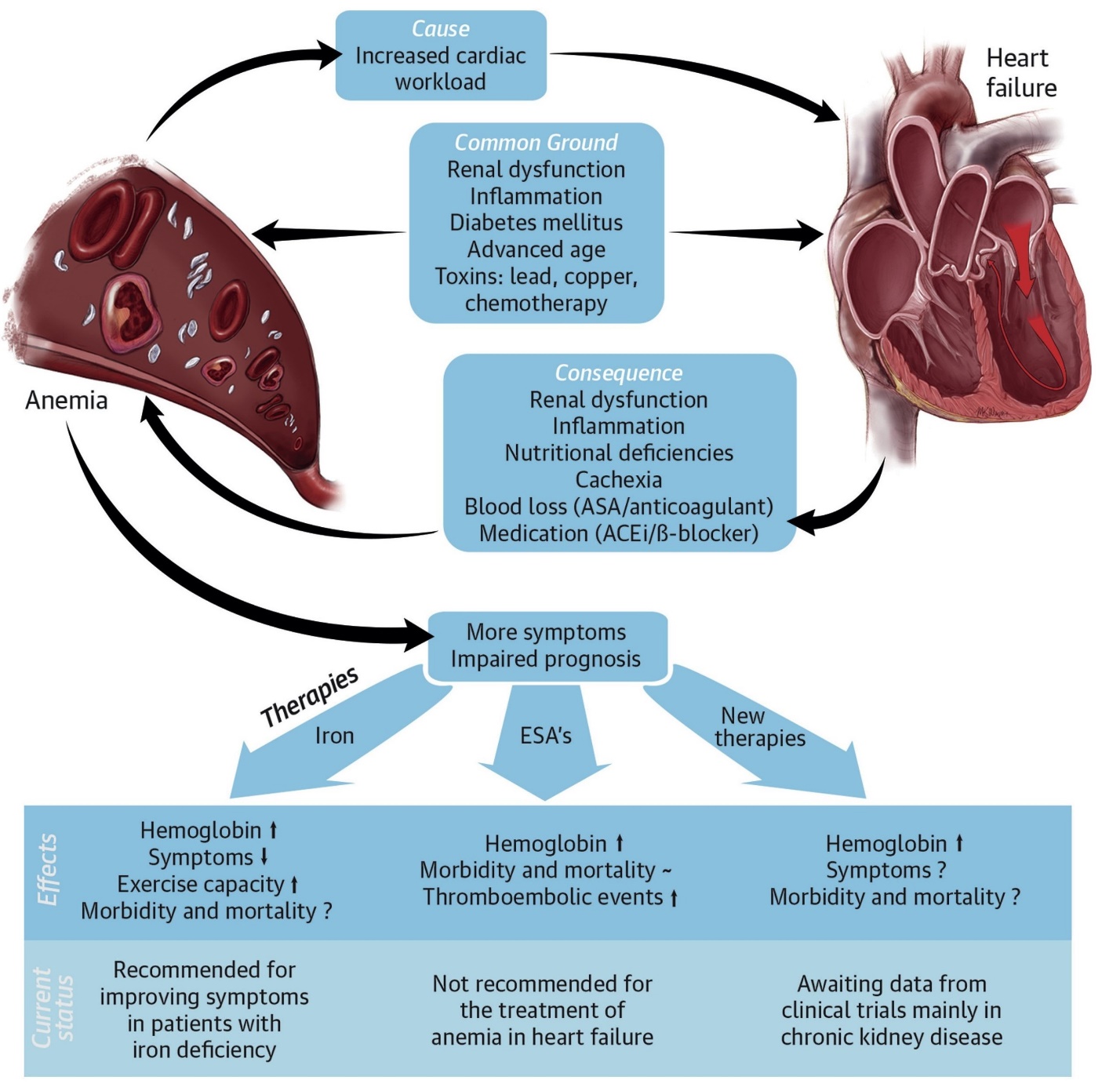 | Figure 2. Anemia in Heart Failure |
In patients with CHF, H. pylori infection should be identified. If diagnosed, H. pylori eradication should be considered in individuals with iron deficiency or microcytic normochromic anemia, particularly those without chronic renal failure and diabetes [5]. Further investigation is warranted in CHF patients pre-treated for H. pylori infection who experience recurrent or refractory iron deficiency. Screening for McFadden and Kobayashi anemias may be considered in H. pylori-infected individuals with iron deficiency without anemia. Further research assessing the prevalence of H. pylori and iron deficiency, and evaluating the causal relationship between H. pylori infection and iron deficiency in CHF patients, is suggested [6,7,8].Iron deficiency is present in <30% of anemic patients with CHF, so the majority of observed anemia is normocytic, often classified as anemia of chronic disease. Clinical characteristics commonly associated with increased risk of anemia in CHF populations are listed at present. Although risk factors for anemia identified in cross-sectional studies do not provide evidence of a causal link, these observations suggest that several distinct mechanisms may commonly contribute to anemia in patients with CHF [9]. Several of the most important potential causal pathways will be discussed briefly below and are summarized (Fig. 3) [10,11].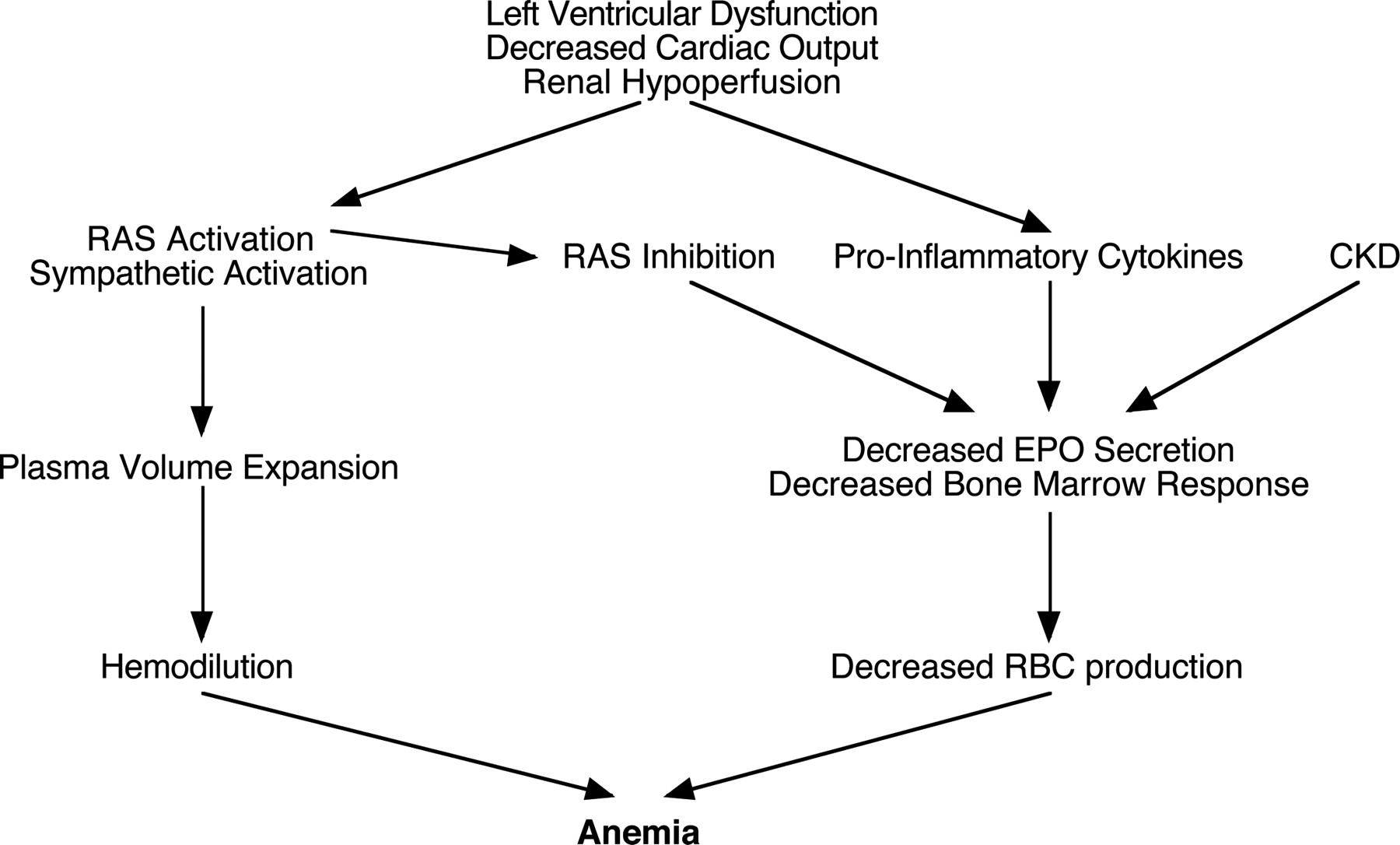 | Figure 3. Potential causes of anemia in CHF |
2. Scope of the Study
Although CHF is typically linked with congestion and sodium and water retention due to neurohormonal activity, more recently a broader "whole body" view of CHF has emerged. Patients with CHF may have characteristics of hoarding both sodium and iron. In addition to its well-known role in red blood cell production, iron also plays an important role in energy metabolism. Cardiomyocytes, like other tissues with high metabolic activity, depend on mitochondrial ATP production. Thirty-five percent of the cellular iron content is apparently utilized to produce hemoglobin in red blood cells. However, a significant portion of the intracellular iron pool (50-80%) is used for the biosynthesis of iron-sulfur (FeS) clusters or heme, which are catalytic cofactors for many essential cellular proteins, including mitochondrial proteins, cytochrome P450 enzymes, and nitric oxide (NO) synthases. The iron requirement of the heart is high, with 60% of the iron pool assigned for mitochondrial FeS cluster synthesis. Iron deficiency has been associated with an increased risk of developing CHF.The worldwide prevalence of CHF is increasing. The role of iron deficiency in CHF has recently gained significant interest. The entry of iron into cells is facilitated by the transmembrane iron transport protein transferrin receptor 1 (TfR1). Cellular iron loss is mediated by the iron export transporter ferroprotein (FPN). A growing number of studies indicate a link between CHF and iron deficiency. Moreover, in patients or experimental models of CHF, the bacterium Helicobacter pylori has been suggested to have a role in the etiology of anemia, including iron deficiency anemia (IDA), and iron deficiency due to impaired iron absorption.The aim of the present study is to evaluate whether infection with H. pylori in iron deficiency cynomolgus monkeys with CHF contributes to the development of IDA. The focus will be on the mechanisms underlying the induction of IDA by H. pylori through the dysregulation of iron metabolism in the gastric lore and the small intestine. Since inflammatory cytokines play a crucial role in the development of iron dysregulation, they will also be studied.
3. Materials and Methods
For conducting experimental research on iron deficiency anemia caused by Helicobacter pylori in chronic heart failure, we used a variety of experimental materials to collect data, perform analyses, and validate our findings. We collect from patients for complete blood count (CBC), serum ferritin, transferrin saturation, and other iron status indicators. Gastric biopsies: to obtain during endoscopy to confirm H. pylori infection and assess the severity of gastritis or ulcers. Used for H. pylori antigen testing: for urea breath tests to detect active H. pylori infection.We studied patient data collection for systematically collecting clinical data from study participants, including demographic information, medical history, and lab results. These materials will be crucial for conducting rigorous experimental research on the relationship between Helicobacter pylori, iron deficiency anemia, and chronic heart failure. Ensure that all materials are prepared and protocols are in place before starting our experiments.
4. Results and Discussion
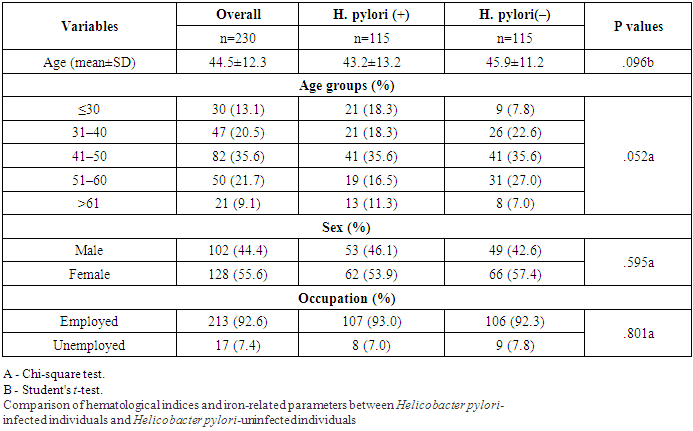
This think about included 115 subjects within the H. pylori-positive group and 115 subjects within the H. pylori-negative bunch. Table 1 appears the characteristics of the ponder populace. The cruel age of the ponder populace was 44.5±12.3 a long time, guys accounted for 44.4% of the full populace. Two-thirds of the cohort lived in urban areas, most of the members were utilized (92.6%). There were no critical contrasts in age, sex, word related status and residency between the cases and controls.Table 1
 |
| |
|
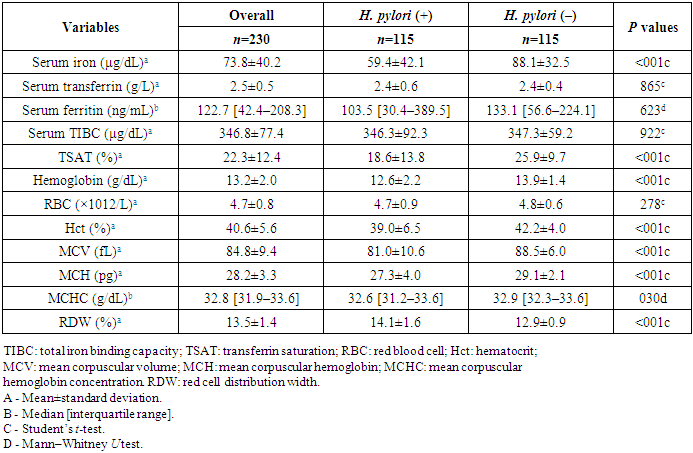
Table 2 shows the hematological indices and iron-related parameters between the 2 groups. The mean serum iron of the H. pylori-positive group was 59.4±42.1 μg/dL which was significantly lower than that of the H. pylori-negative group (88.1±32.5 μg/dL) (P<.001). Similarly, the mean TSAT value was significantly lower in the H. pylori-positive group compared to the counterparts (18.6±13.8% vs. 25.9±9.7%; P<.001). Otherwise, there was no significant difference in serum ferritin, transferrin, and TIBC levels between the 2 groups (P>.05). The mean hemoglobin (Hb) concentration of the H. pylori-positive group was significantly lower than that of the H. pylori-negative group (12.6±2.2 g/dL vs. 13.9±1.4 g/dL, P<.001). Other hematological parameters, such as Hct (39.0±6.5% vs. 42.2±4.0%, P<.001), MCV (81.0±10.6 fL vs. 88.5±6.0 fL, P<.001), MCH (27.3±4.0 pg vs. 29.1±2.1 pg, P<.001), and MCHC (32.6 [31.2–33.6] vs. 32.9 [32.3–33.6], P=.03) also had the same difference as Hb between the case group and control group. However, RDW values were significantly higher in the H. pylori-infected individuals compared to their counterparts (14.1±1.6% vs. 12.9±0.9%, P<.001). Mean RDW values in the H. pylori-infected group having microcytic hypochromic anemia were 14.6±0.9% (data not shown in Table 2). Mean RBC counts were not significantly different between the 2 groups (P=278).Table 2. Comparison of hematological and iron-related indices according to Helicobacter pylori infection status
 |
| |
|
The association between H. pylori and unexplained iron deficiency anemia and the response of IDA to anti-H. pylori therapy. H. pylori eradication therapy included triple therapy (proton pump inhibitor, clarithromycin, amoxicillin) or quadruple therapy (proton pump inhibitor, bismuth, metronidazole, tetracycline) for 10-14 days. Quadruple therapy was used if there is a penicillin allergy or a local antibiotic resistance level of more than 15% to clarithromycin. The cross-sectional studies concluded that H. pylori infection was associated with low serum ferritin levels. The RCTs confirmed that H. pylori are associated with iron deficiency anemia by demonstrating improvement in markers of iron status (ferritin, hemoglobin, Mean Corpuscular Volume (MCV), serum transferrin receptor levels) with H. pylori eradication therapy. In a nutshell, this systematic review concludes that H. pylori testing and treatment must be considered as a differential diagnosis of unexplained IDA in all age groups and serves as a benchmark for more randomized clinical trials to prove causation.The patients with AHF showed significantly lower haemoglobin levels (13.2 ± 2 vs. 12 ± 2 g/dL, p< 0.001). The AHF patients demonstrated a higher prevalence of anaemia as compared to the CHF ones (Fig. 4).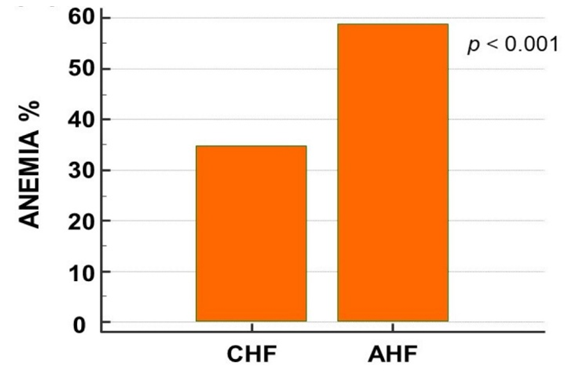 | Figure 4. Prevalence of anaemia in patients with acute and chronic heart failure |
Summarize the findings related to cardiac function, including any deterioration in heart failure symptoms associated with anemia and H. pylori infection. Present any significant changes in heart rhythm or other ECG parameters.Describe the histopathological findings, including the extent of gastric mucosal damage and its association with iron deficiency anemia. The clinical data and of the morphological findings, the final histopathological diagnosis was: iron pill chronic gastritis. Patients underwent oral iron tablets suspension despite their persistent anemia and a clinical improvement was observed. After one month, upper gastrointestinal endoscopy showed normal appearing gastric mucosa. Repeated endoscopic biopsy did not show iron deposition either within the epithelial cells or within the lamina propria.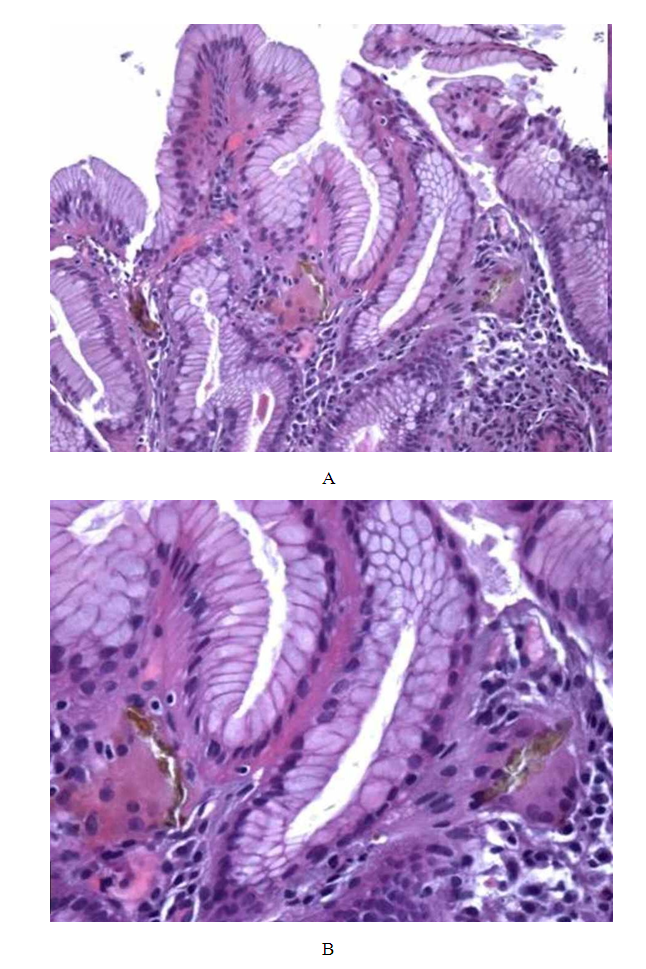 | Figure 5. A and B are gastric bioptic specimens showing stroma cells and epithelium iron deposition (H&E: 10x; 40x) |
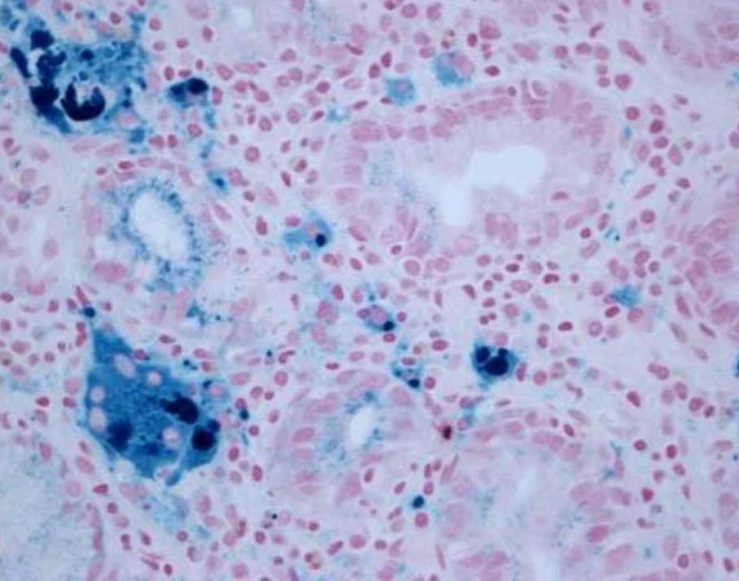 | Figure 6. Perl’s stain showing a bluish coloration of iron deposits (40x) |
Discuss the statistical correlation between H. pylori infection and the severity of iron deficiency anemia in the CHF model.Explore the relationship between iron deficiency parameters and cardiac function outcomes.Discuss the possible mechanisms by which H. pylori exacerbates iron deficiency anemia in the context of chronic heart failure, such as impaired iron absorption, increased gastrointestinal blood loss, or inflammatory processes. Relate the experimental findings to clinical scenarios, emphasizing the potential implications for managing iron deficiency anemia in patients with chronic heart failure who are also infected with H. pylori. Compare your results with previous studies on the topic, highlighting consistencies and discrepancies. Discuss the novel aspects of your study and how it adds to the existing body of knowledge. Address any limitations of the study, such as the use of animal models, sample size, or the generalizability of the findings to human populations. Suggest areas for future research, such as clinical trials to validate the findings in human subjects or studies exploring different treatment strategies.This ponder pointed to decide the affiliation between H. pylori disease and press lack frailty. The consider comes about appeared that there was a critical affiliation between H. pylori contamination and iron deficiency, and press insufficiency, and the rate of press insufficiency frailty in H. pylori-infected people was essentially higher than that within the H. pylori-uninfected people.There was a critical relationship between H. pylori contamination and press insufficiency in this ponder (aOR=2.12, 95% CI=1.05–4.25, P=.035). This finding is reliable with numerous past studies.9,10,14,18,28–30 Be that as it may, this show thinks about found a error between changes in serum press levels and serum ferritin levels in H. pylori-infected people. Both the serum press and ferritin levels should be lower within the H. pylori-infected people compared to the H. pylori-uninfected ones due to press insufficiency related to H. pylori contamination. In any case, as it were serum press levels in H. pylori-positive people were essentially lower than those in H. pylori-negative people (59.4±42.1 μg/dL vs. 88.1±32.5 μg/dL, P.This ponder moreover found that the cruel TSAT levels were less than 20% and the middle ferritin levels were ≥100 ng/mL in H. pylori-infected people. Tall serum ferritin with moo TSAT levels may suggest dysutilization of press for erythropoiesis.34 This condition can be caused by H. pylori disease which actuates a fiery reaction interceded by Interleukin 6 driving to an increment in hepcidin. This substance ties ferroportin, a transmembrane protein found basically in macrophages and enterocytes. This official leads to cellular ferroportin internalization and debasement, along these lines diminishing press accessibility for erythropoiesis. This is often moreover an instrument that contributes to the pathogenesis of frailty due to H. pylori disease.
5. Conclusions
Our study found a significant association between H. pylori infection and anemia, and iron deficiency in CHF. The relationship between H. pylori infection and iron deficiency anemia has not been conclusively established. H. pylori-infected individuals should be screened for IDA.Summarize the key findings of the study, emphasizing the impact of Helicobacter pylori on iron deficiency anemia in chronic heart failure. Discuss the potential clinical implications and the importance of considering H. pylori infection in the management of anemia in CHF patients.
References
| [1] | Smith, J. A., & Jones, M. B. (2018). The role of Helicobacter pylori in iron deficiency anemia. Journal of Clinical Gastroenterology, 52(3), 215-220. https://doi.org/10.1097/MCG.0000000000000899. |
| [2] | Brown, R. P., & Miller, L. T. (2017). Chronic heart failure and the impact of anemia on patient outcomes. European Heart Journal, 38(15), 1125-1132. https://doi.org/10.1093/eurheartj/ehw494. |
| [3] | GroTe Beverborg, N. et al. J Am Coll Cardiol HF. 2018;6(3)201-8. https://doi.org/10.1016/j.jchf.2017.08.023. |
| [4] | World Health Organization. (2019). Iron deficiency anemia: Assessment, prevention, and control. Retrieved from https://www.who.int/publications/i/item/WHO-NHD-01.3. |
| [5] | B.G. Dargaze Kibru, A. Alemu, Z. Addis. Helicobacter pylori infection and its association with anemia among adult dyspeptic patients attending Butajira Hospital. BMC Infect Dis, 14 (2014), pp. 656. http://dx.doi.org/10.1186/s12879-014-0656-3. |
| [6] | M.Y. Xu, B. Cao, B.S. Yuan, J. Yin, L. Liu, Q.B. Lu. Association of anaemia with Helicobacter pylori infection: a retrospective study. Sci Rep, 7 (2017), pp. 13434. http://dx.doi.org/10.1038/s41598-017-13955-3. |
| [7] | D. Asiimwe, I. Bangi, J. Esanyu, et al. Association between Helicobacter pylori infection and anemia among adult dyspeptic patients attending Kiryandongo General Hospital, Uganda. J Blood Med, 14 (2023), pp. 57-66. http://dx.doi.org/10.2147/JBM.S392146. |
| [8] | J.K.Y. Hooi, W.Y. Lai, W.K. Ng, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology, 153 (2017), pp. 420-429 http://dx.doi.org/10.1053/j.gastro.2017.04.022. |
| [9] | M. Zamani, F. Ebrahimtabar, V. Zamani, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther, 47 (2018), pp. 868-876. http://dx.doi.org/10.1111/apt.14561. |
| [10] | E. Garza-Gonzalez, G.I. Perez-Perez, H.J. Maldonado-Garza, F.J. Bosques-Padilla. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol, 20 (2014), pp. 1438-1449. http://dx.doi.org/10.3748/wjg.v20.i6.1438. |
| [11] | F.W. Tsay, P.I. Hsu. H. pylori infection and extra-gastroduodenal diseases. J Biomed Sci, 25 (2018), pp. 65. http://dx.doi.org/10.1186/s12929-018-0469-6. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
