Nodir Ruzimurodov1, Tamara Aripova2, Diloram Musakhodjayeva3, Zukhra Azizova4
1PhD, Scientific Secretary, Institute of Immunology and Human Genomics, Tashkent, Uzbekistan
2DSc, Director, Institute of Immunology and Human Genomics, Tashkent, Uzbekistan
3DSc, Head of the Laboratory, Institute of Immunology and Human Genomics, Tashkent, Uzbekistan
4PhD, Senior Researcher, Institute of Immunology and Human Genomics, Tashkent, Uzbekistan
Correspondence to: Nodir Ruzimurodov, PhD, Scientific Secretary, Institute of Immunology and Human Genomics, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This paper presents the results of a study of the immune status in adult patients with diabetes mellitus in the postcovid period. The levels of key cytokines such as VEGF-A, TGF-β, IGF-1, IP-10, and adhesion molecules VCAM-1 and ICAM-1 were analyzed. The study includes a comparison between vaccinated and unvaccinated patients, revealing vaccination's effect on inflammatory processes and metabolic parameters.
Keywords:
Immune status, Diabetes Mellitus, Postcovid Period, Cytokines
Cite this paper: Nodir Ruzimurodov, Tamara Aripova, Diloram Musakhodjayeva, Zukhra Azizova, Immunological Status in Diabetes Mellitus in the Adult Population in the Postcovid Period, American Journal of Medicine and Medical Sciences, Vol. 14 No. 10, 2024, pp. 2659-2662. doi: 10.5923/j.ajmms.20241410.44.
1. Introduction
Relevance of the study: The COVID-19 pandemic has significantly impacted the study of the immune system in patients with diabetes mellitus (DM). Patients with DM have a higher risk of severe COVID-19, which is associated with chronic inflammation and dysfunction of endothelial cells. COVID-19 impairs glycemic control, increasing the risk of complications in diabetic patients due to inflammatory processes and impaired immune response. The purpose of the study is to study immunological parameters in patients with DM who have suffered a coronavirus infection, with an emphasis on the effect of vaccination against COVID-19 on the immune status. Methods: The study was conducted among three groups of patients and a control group of practically healthy individuals. The first group included 33 patients with diabetes diagnosed before the pandemic. Immunological studies were conducted using ELISA methods. Results: IGF-1 levels were significantly higher in diabetic patients compared to the control group. In the first group, the average IGF-1 level was 613.37±17.50 ng/ml, which is almost 6 times higher than in the control group. The IP-10 level was also significantly increased in diabetic patients, reaching 541.11±15.18 pg/ml, which is more than 2.6 times higher than the control values. The levels of adhesion molecules VCAM-1 and ICAM-1 showed a significant increase in the group of unvaccinated patients, indicating severe endothelial dysfunction and chronic inflammation. Conclusion: The results show that diabetic patients have marked changes in immune and metabolic processes after COVID-19. Vaccination improves the immune response and reduces the risk of complications associated with inflammatory reactions and endothelial disorders.The COVID-19 pandemic has significantly impacted research approaches to the study of the immune system in patients with diabetes mellitus (DM). Studies show that patients with DM have a higher risk of severe COVID-19, which is associated with chronic inflammation and endothelial cell dysfunction. COVID-19 may worsen glycemic control and increase the risk of complications in patients with diabetes due to inflammatory processes and impaired immune response. Vaccination against COVID-19 has shown the ability to improve the immune response in patients with diabetes, reducing the risk of severe disease and hospitalization. Immunological studies in type 2 diabetes mellitus (T2DM) are important because chronic inflammation and immune dysfunction play a key role in the pathogenesis and progression of this disease. Patients with DM are at increased risk of infections and inflammatory diseases, which exacerbates their condition and leads to the development of complications. In the context of the COVID-19 pandemic, the study of the immunological status of patients with diabetes becomes even more relevant, since coronavirus infection causes pronounced immunoactivation and can significantly worsen the metabolic state and increase the risk of complications in this category of patients.The purpose of the study: In our study, immunological parameters were studied in patients with DM in a previous coronavirus infection and vaccination against COVID-19. Due to limited resources and the similarity of clinical and laboratory parameters in different groups of patients, we focused on representative samples.
2. Materials and Methods
Immunological studies were conducted within the framework of three main groups of patients and a control group of practically healthy individuals. The first and main group included 33 patients with diabetes diagnosed before the pandemic outbreak. The control group included 35 practically healthy people, comparable in age, gender and absence of chronic diseases, which allowed minimizing the influence of these factors on the results of the study.
3. Results and Discussion
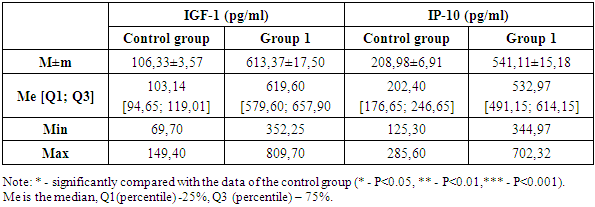
In diabetes mellitus and COVID-19, special attention is paid to the study of immunological characteristics, since both pathologies are associated with pronounced changes in the immune system. Diabetes mellitus is associated with chronic inflammation and dysfunction of immune cells, which increases the risk of severe infections. COVID-19, in turn, can provoke a hyperinflammatory reaction known as a "cytokine storm", which can lead to serious complications and deterioration of the condition of patients with diabetes. Studying the immunological features of these two pathologies helps to understand how SARS-CoV-2 infection interacts with metabolic disorders in diabetes, which may contribute to the development of more effective methods of treatment and prevention of complications. It is important to discuss key biomolecules such as IGF-1 (insulin-like growth factor 1) and IP-10 (inducible protein 10 kDa), which play a significant role in immune and metabolic processes, especially in diseases such as diabetes mellitus and COVID-19 (Table 1).Table 1. IGF-1 and IP-10 indicators in the study groups
 |
| |
|
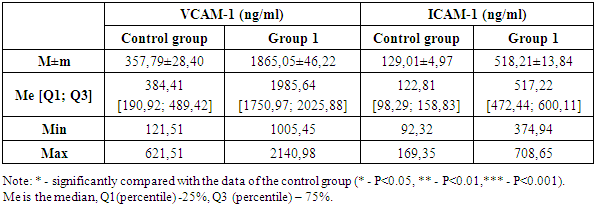
IGF-1 is an important mediator of growth and metabolism, closely related to the insulin system. It is involved in the regulation of cell growth, differentiation and survival, and supports metabolic homeostasis. In diabetes mellitus, there is a violation of IGF-1 signaling pathways, which can lead to metabolic dysfunctions, delayed wound healing and other complications associated with diabetes. In COVID-19 conditions, changes in IGF-1 levels can affect the body's response to infection and the severity of the disease. Studying IGF-1 in these pathologies allows us to understand how metabolic disorders can interact with the immune system, affecting the outcomes of the disease.In the first group, IGF-1 levels were significantly higher compared to the control group. The average IGF-1 value in the first group was 613.37±17.50 ng/ml, which is almost 6 times higher than in the control group, where the average IGF-1 level was 106.33±3.57 ng/ml. The median IGF-1 level in the first group was 619.60 ng/ml, whereas in the control group this indicator was significantly lower and amounted to 103.14 ng/ml. This indicates a significant difference in the distribution of IGF-1 values between the two groups. The interquartile range in the first group varied within [579.60; 657.90] ng/ml, reflecting a wider variability in IGF-1 levels among patients with diabetes mellitus. In the control group, the interquartile range was [94.65; 119.01] ng/ml, indicating relatively stable levels of this marker among healthy people. The minimum and maximum values in the first group also show a significant deviation from the control group. In the first group, the minimum IGF-1 value was 352.25 ng/ml, and the maximum was 809.70 ng/ml. In the control group, these indicators were significantly lower: the minimum value was 69.70 ng/ml, the maximum was 149.40 ng/ml. These data highlight the severity of metabolic disorders in patients with diabetes mellitus and a significant increase in IGF-1 levels compared to the control group. The difference between the mean and median in both groups indicates a symmetrical distribution of data, despite high IGF-1 levels in patients with DM.The next step of the study was to measure the level of IP-10 in the control group and in patients with diabetes mellitus. The level of IP-10 in the first group of patients with diabetes was significantly increased compared to the control group. The average IP-10 level in diabetic patients was 541.11±15.18 pg/ml, which is more than 2.6 times higher than the average of the control group, equal to 208.98±6.91 pg/ml.The median IP-10 level also showed significant differences: in patients with diabetes, it reached 532.97 pg/ml, whereas in healthy participants in the control group, the median was 202.40 pg/ml.The analysis of the interquartile range revealed wider boundaries in patients with diabetes — [491.15; 614.15] pg/ml, in contrast to the control group, where the interquartile range was within [176.65; 246.65] pg/ml. This indicates a greater variability in IP-10 levels in patients with DM.As for the minimum and maximum values of IP-10, in the first group they were 344.97 pg/ml and 702.32 pg/ml, respectively, while in the control group this range was significantly lower — from 125.30 pg/ml to 285.60 pg/ml. These findings highlight the severity of the inflammatory response in patients with diabetes mellitus. The difference between the mean and median in both groups indicates a symmetrical distribution of data, despite high levels of IP-10 in patients with DM.A study of IGF-1 and IP-10 levels in patients with DM in COVID-19 demonstrates a significant increase in these markers compared to the control group. This indicates an increase in metabolic disorders and inflammatory processes in this category of patients, which emphasizes the need for careful monitoring and correction of these parameters to improve clinical outcomes. Elevated levels of IGF-1 and IP-10 in combination with chronic inflammation in DM create prerequisites for severe complications, especially in the context of the COVID-19 pandemic.For a deeper understanding of the pathophysiological processes occurring in diabetes mellitus and its complications, the next stage of our study was the study of cell adhesion molecules such as VCAM-1 (vascular cell adhesion molecule 1) and ICAM-1 (intercellular adhesion molecule 1) (Table 2). Table 2. VCAM-1 and ICAM-1 indicators in the study groups
 |
| |
|
VCAM-1 and ICAM-1 are important for understanding the pathogenesis of inflammatory and vascular complications in diabetes mellitus. These molecules play a key role in regulating interactions between endothelial cells and leukocytes, which is critical for the development of inflammatory processes and atherosclerosis. VCAM-1 and ICAM-1 are integral membrane proteins that are expressed on the surface of endothelial cells in response to pro-inflammatory cytokines such as TNF-α and IL-1. They are involved in the attachment of circulating leukocytes to endothelial cells, which is the initial stage of their migration into tissues where inflammation occurs. In diabetes mellitus, chronic inflammation and hyperglycemia stimulate an increase in the levels of VCAM-1 and ICAM-1, which leads to an increase in the adhesion of monocytes and other leukocytes to the vascular endothelium. This contributes to the activation of the endothelium, the penetration of inflammatory cells into the vascular wall and the development of atherosclerotic plaques. The increased expression of these molecules is a marker of endothelial dysfunction, which is closely associated with the development of complications such as coronary heart disease, stroke and peripheral arterial disease in patients with diabetes. In addition, elevated levels of VCAM-1 and ICAM-1 can enhance vascular permeability, which contributes to plasma leakage and the development of edema, thus enhancing the pathological process.In the course of the study, the levels of VCAM-1 were analyzed among the control group and the group of patients with diabetes mellitus who did not receive vaccination. In the control group, the average level of VCAM-1 was 357.79±28.40 ng/ml, while the median value was 384.41 ng/ml, and the interquartile range ranged from 190.92 to 489.42 ng/ml. The minimum and maximum values of VCAM-1 in the control group ranged from 121.51 ng/ml to 621.51 ng/ml, which indicates a fairly stable distribution of this marker among healthy participants.In the group of patients with diabetes mellitus who did not receive vaccination, there was a significant increase in VCAM-1 levels. The average level of VCAM-1 in this group reached 1865.05±46.22 ng/ml, which is more than 5 times higher than the same indicator in the control group. The median value was 1985.64 ng/ml, with an interquartile range from 1750.97 to 2025.88 ng/ml. The minimum and maximum values in this group ranged from 1005.45 ng/ml to 2140.98 ng/ml, indicating significant variability in VCAM-1 levels among diabetic patients.The difference between the mean and median in both groups deserves special attention. In the control group, the mean value (357.79 ng/ml) and the median (384.41 ng/ml) are in a close range, which indicates a symmetrical distribution of data. In the group of patients with diabetes, a similar situation is observed when comparing the mean (1865.05 ng/ml) and median (1985.64 ng/ml), which also indicates a symmetrical distribution of data, despite higher indicators.VCAM-1 is known to be a marker of endothelial activation, which plays a critical role in the development of inflammatory processes and atherosclerosis. Its mechanism of action includes activation of endothelial cells, which promotes adhesion of monocytes and other leukocytes to the vascular wall, enhancing the inflammatory response and contributing to the progression of atherosclerotic changes. In patients with diabetes mellitus, elevated levels of VCAM-1 indicate significant endothelial dysfunction and chronic inflammation, which is especially important in COVID-19 conditions, when the risk of vascular complications increases significantly. These data highlight the importance of monitoring VCAM-1 levels to assess the risk of developing vascular diseases and optimize therapeutic approaches in patients with diabetes mellitus.ICAM-1 is a key biomarker of inflammatory processes and plays a significant role in the pathogenesis of various diseases. ICAM-1 is expressed on the surface of endothelial and immune cells, contributing to the attachment of leukocytes and their subsequent migration to the focus of inflammation. In patients with diabetes mellitus, chronic inflammation and endothelial dysfunction lead to increased levels of ICAM-1, and infection with SARS-CoV-2 significantly exacerbates these processes. The COVID-19 virus causes massive activation of the immune system and damage to the endothelium, which leads to an additional increase in ICAM-1 levels and increases inflammatory reactions.A study of ICAM-1 levels in the control group demonstrated that the average value of this marker was 129.01±4.97 ng/ml. The median value was at the level of 122.81 ng/ml, and the interquartile range was [98.29; 158.83] ng/ml. The minimum and maximum values in the control group ranged from 92.32 ng/ml to 169.35 ng/ml, which indicates the relative stability of ICAM-1 levels among healthy participants.In group 1, the ICAM-1 level was significantly increased. The average value of ICAM-1 was 518.21±13.84 ng/ml, which is 4 times higher than in the control group. The median in this group was also significantly higher and amounted to 517.22 ng/ml. The interquartile range ranged from 472.44 to 600.11 ng/ml, indicating significant variability in ICAM-1 levels among diabetic patients. The minimum values of ICAM-1 in this group were 374.94 ng/ml, and the maximum values were 708.65 ng/ml, reflecting pronounced changes in ICAM—1 levels in diabetic patients.The difference between the mean and median in both groups deserves special attention. In the control group, the mean value of ICAM-1 (129.01 ng/ml) and the median (122.81 ng/ml) are in a close range, indicating a symmetrical distribution of data. In the group of patients with diabetes, a similar situation is observed when comparing the mean (518.21 ng/ml) and median (517.22 ng/ml), which also indicates a symmetrical distribution of data, despite higher ICAM-1 values.
4. Conclusions
Thus, our study revealed significant changes in the levels of key immunological and biochemical markers in patients with type 2 diabetes mellitus, especially in concomitant infections such as COVID-19. It was found that the levels of IGF-1, IP-10, VCAM-1, and ICAM-1 were significantly increased compared to the control group, indicating an increase in inflammatory and vascular disorders. At the same time, the level of adiponectin was significantly reduced, indicating a loss of its anti-inflammatory and metabolic protective properties. These results highlight the importance of a comprehensive approach to monitoring and managing the condition of diabetic patients, especially in a pandemic, in order to prevent the development of serious complications and improve the prognosis.
References
| [1] | Apicella, M., Campopiano, M. C., Mantuano, M., Mazoni, L., Coppelli, A., & Del Prato, S. (2020). COVID-19 and diabetes: How high is the risk? Diabetes Research and Clinical Practice, 162, 108137. |
| [2] | Ayanoglu, F.B.; Elçin, A.E.; Elçin, Y.M. Bioethical issues in genome editing by CRISPR-Cas9 tec technology. Turk. J. Biol. 2020, 44, 110–120. |
| [3] | Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress. |
| [4] | Bornstein, S. R., Dalan, R., Hopkins, D., Mingrone, G., Boehm, B. O., & Balancing, A. (2020). Endocrine and metabolic link to coronavirus infection. Nature Reviews Endocrinology, 16, 297-298. https://doi.org/10.1038/s41574-020-0353-9. |
| [5] | Ceriello, A., De Nigris, V., & Prattichizzo, F. (2020). COVID-19: Glucotoxicity, lipidotoxicity and hypoxia. Could diabetes worsen COVID-19 outcomes? Diabetes Research and Clinical Practice, 163, 108151. https://doi.org/10.1016/j.diabres.2020.108151. |
| [6] | Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener- Kronish, J.P. |
| [7] | Muniyappa, R., & Gubbi, S. (2020). COVID-19 pandemic, coronaviruses, and diabetes mellitus. American Journal of Physiology-Endocrinology and Metabolism, 318(5), E736-E741. https://doi.org/10.1152/ajpendo.00124.2020. |
| [8] | Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015; 7: 449-490. |
| [9] | Slezak J. Rate and severity of suspected SARS-Cov-2 reinfection in a cohort of PCR-positive COVID-19 patients // Clin. Microbiol. Infect. 2021. Vol. 27, № 12. P. 1860. e7–1860.e10. |
| [10] | Valdez-Cruz N.A. Integrative overview of antibodies against SARS-CoV- 2 and their possible applications in COVID-19 prophylaxis and treatment // Microb. Cell Fact. 2021. Vol. 20, № 1. P. 88. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
