Panjieva N. N., Khaydarov N. K., Raimova M. M.
Tashkent State Dental Institute, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Chemotherapy-induced polyneuropathy (CIPN) is a significant side effect of chemotherapy, particularly affecting ovarian cancer patients. This study aimed to examine the clinical features and progression of CIPN in women undergoing chemotherapy for ovarian cancer. The study included 110 patients receiving chemotherapy. Participants were divided into two groups: those with subjective and objective symptoms of polyneuropathy and those with only objective symptoms. Various scales and questionnaires, including NCI-CTCAE, LANSS, and FACT/GOG-Ntx, were used for assessment. Results demonstrated that CIPN is accompanied by sensory and motor impairments significantly affecting daily activities and quality of life. Main symptoms included numbness, pain, and sensory disturbances in the extremities. Objective neurological examination confirmed the presence of sensory, motor, and autonomic deficits in CIPN patients. This study helps to better understand the nature and severity of CIPN in ovarian cancer patients, potentially aiding in optimizing treatment and improving their quality of life.
Keywords:
Chemotherapy-induced polyneuropathy
Cite this paper: Panjieva N. N., Khaydarov N. K., Raimova M. M., Clinical Features and Course of Chemotherapy-Induced Polyneuropathy in Women with Ovarian Cancer, American Journal of Medicine and Medical Sciences, Vol. 14 No. 10, 2024, pp. 2529-2532. doi: 10.5923/j.ajmms.20241410.17.
1. Introduction
Chemotherapy-induced polyneuropathy (CIPN) is a common and severe side effect of chemotherapy, particularly in ovarian cancer patients. This condition is characterized by peripheral nerve damage, leading to sensory and motor disturbances like tingling, numbness, and pain in the extremities. Ovarian cancer, a frequently diagnosed gynecological malignancy, requires aggressive chemotherapy regimens, often resulting in severe peripheral nerve damage. [1]According to the World Health Organization (WHO), ovarian cancer accounts for approximately 37% of all cancer cases in women annually, making it a leading cause of cancer mortality globally. Late diagnosis and the disease's latent nature contribute to high mortality rates despite advancements in treatment and screening. [3]Chemotherapy is the primary treatment for ovarian cancer. Platinum-based drugs such as cisplatin and carboplatin, combined with taxanes like paclitaxel, are standard treatments. These drugs have significantly improved survival rates but are associated with severe side effects, including CIPN. The neurotoxic effect of these chemotherapeutic agents can lead to persistent and sometimes irreversible peripheral nerve damage. [4]Purpose of the study. To investigate the clinical features and progression of chemotherapy-induced polyneuropathy in women receiving chemotherapy for ovarian cancer.
2. Materials and Methods
The study was conducted in the "Female Reproductive System Tumors" department at the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology. The study included 110 patients diagnosed with ovarian cancer and undergoing chemotherapy from January 2022 to May 2023. Patients were divided into two groups: the first group included 70 patients with subjective and objective symptoms of polyneuropathy, and the second group included 40 patients with only objective symptoms. Additionally, 30 healthy volunteers were included as a control group. All patients received chemotherapy with platinum and taxane drugs. [2]Participants were selected based on the following criteria: women aged 18 to 80 years, histologically confirmed diagnosis of ovarian cancer, planned chemotherapy of at least two months, presence of subjective and/or objective symptoms of polyneuropathy, and signed informed consent. Exclusion criteria included diseases affecting study results (diabetes, pre-existing polyneuropathy), brain and bone metastases, unstable psycho-emotional state, and drug intolerance. Each patient underwent a detailed medical history and a complete clinical and neurological examination, including sensory, motor, and autonomic function assessments, and using scales and questionnaires such as NCI-CTCAE, LANSS, and FACT/GOG-Ntx.Standard statistical methods, including descriptive statistics and correlation analysis, were used for data analysis. The significance level was set at p<0.05. Patients received standard ovarian cancer chemotherapy regimens, including platinum-based drugs (cisplatin or carboplatin) and taxanes (paclitaxel or docetaxel). The choice of regimen was based on clinical guidelines and oncologist discretion.Demographic data, cancer stage, chemotherapy regimen, and dosage were collected. CIPN severity was assessed using CTCAE version 5.0. Baseline characteristics of the study population, including age, cancer stage, and comorbidities, were recorded and analyzed. [5]
3. Results and Discussion
The average age of participants was 51.5±7.74 years in the first group, 53.4±8.93 years in the second group, and 52.1 years in the control group. Middle-aged women predominated (50%), followed by elderly (29%), young (20%), and advanced age (0.9%). The distribution by age groups is shown.Among participants, 63% had stage I ovarian cancer, 13.6% stage II, 75.4% stage III, and 4.5% stage IV cancer.Interviews revealed that besides fatigue, general weakness, and anxiety (possibly related to laboratory blood test changes such as anemia and increased ESR), the most common neurological complaints were sensory and motor disturbances. Among ovarian cancer patients with both subjective and objective symptoms of polyneuropathy before treatment, the main subjective complaints included unpleasant sensations in the distal parts of the arms and legs, difficulties in evaluating palpable objects, numbness of fingers and toes, a sensation of crawling ants, heaviness in the feet, and pain when touching cold water. Motor disturbances included difficulty eating, fastening buttons and locks, and putting on earrings. Many patients experienced fear and uncertainty when walking due to unexplained leg sensations and sudden dizziness when standing up quickly.Neurological examination revealed symmetrical sensitivity disturbances, fine motor impairment, and reduced or absent reflexes. No cases of paralysis were observed.Table 1. General characteristics of the clinical study participants
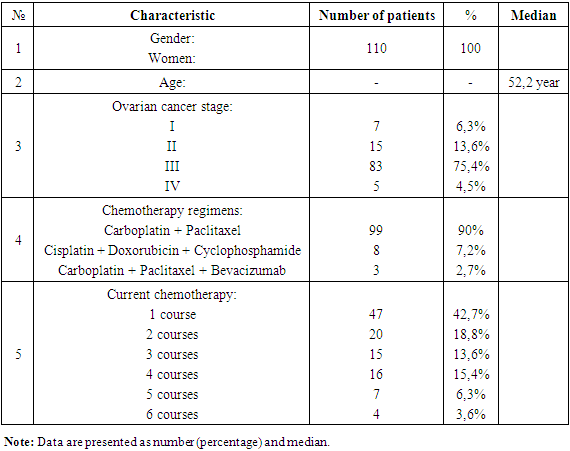 |
| |
|
Among first group patients, sensory disturbances were noted in all participants. In 25.7% of cases, complaints were periodic, while 74.2% had constant complaints. Symptoms included decreased sensitivity, numbness of fingers and toes, and heaviness in feet. Clinical symptoms of neuropathic pain included painful sensations when in contact with cold water and a sensation of electric shock when touching objects. Motor disturbances manifested as difficulty eating, fastening buttons, and putting on earrings. Many patients experienced fear and uncertainty when walking due to unexplained leg sensations and sudden dizziness when standing up quickly.Sensitive impairments were noted in all participants of the first group (n=70). In 25.7% (n=18), these complaints occurred periodically, while in 74.2% (n=52) they were constant. Symptoms of loss (reduced sensitivity, numbness in the fingers of the hands or feet, soles, and palms) were observed in 27.2% (n=19) of patients, of which 18.5% (n=13) were in the lower extremities, 2.8% (n=2) in the upper extremities, and 5.7% (n=4) in both extremities. Irritation symptoms (burning, tingling, a feeling of "crawling ants" in the hands or feet) were noted by 24.2% (n=17) of patients, including 15.7% (n=11) in the lower extremities, 4.2% (n=3) in the upper extremities, and 4.2% (n=3) in both extremities. The remaining 48.5% (n=33) of patients indicated the presence of both irritation and loss symptoms simultaneously: 25.7% (n=18) only in the lower extremities, 14.2% (n=10) only in the upper extremities, and 7.1% (n=5) in both extremities. Thus, complaints of sensitivity impairment only in the distal parts of the upper extremities were found in 22.8% (n=16) of patients, only in the distal parts of the lower extremities in 60% (n=42), and simultaneously in the distal parts of the upper and lower extremities in 17.1% (n=12). Moreover, no patient reported symptoms extending above the elbows or knees.Motor impairments were less common than sensory symptoms. Motor symptoms combined with sensory symptoms were identified in 32.8% (n=23) of patients. Complaints of motor symptoms of loss, such as clumsiness in the fingers and inability to hold small objects, were noted by 22.8% (n=16) of patients, while clumsiness in the feet was noted by 4.3% (n=1). Motor irritation symptoms in the form of muscle cramps in the lower extremities were noted by 13.1% (n=3) of patients, and in the upper extremities by 8.6% (n=2). Additionally, one patient (1.4%) reported simultaneous motor symptoms of loss and irritation in the distal parts of both upper and lower extremities. [6]Autonomic symptoms were very rarely recorded and included sweating disorders, manifested as dry palms in 8.5% (n=6) of patients, and constipation in 15% (n=11). Polyneuropathy symptoms significantly affected patients' daily activities and quality of life. For example, 40% (n=28) of patients reported significant difficulties in walking due to sensory and motor impairments. Additionally, 22.8% (n=16) experienced problems performing tasks requiring fine motor skills, such as buttoning, eating, using a mobile device, or writing. Moreover, 50% (n=35) of patients reported sleep disturbances due to pain or discomfort, worsening their overall condition.Patients in group II did not report subjective complaints of polyneuropathy symptoms; however, all noted general weakness and increased fatigue. Some experienced painful sensations throughout the body, without being able to specify exact locations.Neurological examination showed that 64.2% (n=45) of group I participants had reduced sensitivity to pinpricks, indicating possible dysfunction of peripheral small fiber nerves. Additionally, 48.5% (n=34) of patients showed reduced temperature sensitivity in the distal parts of the lower extremities. Reduced tactile sensitivity in the distal parts of both upper and lower extremities was found in 8.5% (n=6) of patients, only in the distal parts of the lower extremities in 2 patients, and complete absence of tactile sensitivity in the distal parts of the lower extremities in 2 patients. Allodynia (pain from an external stimulus) was found in 27.1% (n=19) of patients, and hyperalgesia (increased sensitivity to pain) was present in 25% (n=18) of patients, making even minor injuries or contact very painful. Clinical examination revealed normative values of vibratory sensitivity in the distal parts of the upper and lower extremities in 68.5% of patients (n=48). Signs of reduced vibratory sensitivity in the distal parts of the upper and lower extremities were observed in 31.4% of patients (n=22). Reduced muscle strength in the distal parts of both upper and lower extremities was not detected in 81.4% of the examined (n=57), but reduced muscle strength in the lower extremities was noted in 18.5% (n=13).Evaluation of deep reflexes revealed symmetrical reduction in carporadial reflexes in 55.7% of patients (n=39), knee reflex in 62.8% (n=44), Achilles reflexes in 74.2% (n=52), and complete suppression of Achilles reflexes in 22.8% (n=16). Muscle hypotrophy, mainly in the small muscles of the hands and feet, was observed in 10% (n=7) of patients.Objective neurological examination of patients with CIPN revealed significant sensory, motor, and autonomic deficits. These objective indicators confirm the subjective symptoms reported by patients and underscore the significant impact of antitumor drugs on the peripheral nervous system.Objective neurological examination of patients in the second group, who did not complain of polyneuropathy symptoms, revealed less pronounced sensory impairments. Reduced sensitivity to pinpricks was found in 70% (n=28) of patients, indicating early dysfunction of small fiber nerves. Reduced temperature sensitivity was noted in 27.5% (n=11), and reduced tactile sensitivity in the distal parts of both upper and lower extremities was found in 5% (n=2) of patients. Impaired vibratory sensitivity, changes in muscle strength, and muscle hypotrophy were not detected in this group. Reduced patellar reflex was found in 27.5% (n=11), and hyporeflexia of the Achilles reflex was noted in 45% (n=18) of patients. Autonomic dysfunction was not detected in this group.Objective neurological examination of patients without complaints of polyneuropathy symptoms revealed early sensory and motor deficits, which were less pronounced compared to the main group. According to the classification by the NCI-CTCAE scale (Common Terminology Criteria for Adverse Events. Changes from the nervous system), grade 2 was identified in 75.7% (n=53) of patients in the first group: symptoms affecting daily activities were observed in these patients. Grade 3 was identified in 24.2% (n=17) of patients, with symptoms significantly affecting their ability to perform daily activities and limiting their activity. Grades 4 and 5 peripheral nervous system neurotoxicity were not detected in the examined patients. All participants in the second group (n=40) were classified as having grade 1 neurotoxicity.Table 2. Peripheral nervous system neurotoxicity
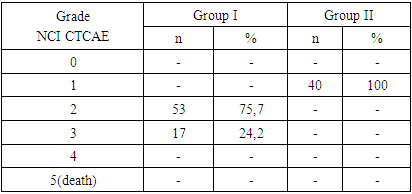 |
| |
|
To objectify the condition of patients with CIPN, their symptoms were assessed using the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group – Neurotoxicity (FACT/GOG-Ntx) scale. In the first group, before the start of treatment, severe symptoms (20-23 points) were observed in 31.4% (n=22) of the patients, moderate severity (16-19 points) in 40% (n=28), and mild severity (12-15 points) in 28.5% (n=20). In the second group, due to the absence of subjective complaints, the assessment on this scale did not show any changes. The average score for functional assessment of neurological deficit using the FACT/GOG-Ntx scale in the first group was 18.01±2.53, while in the second group it was 2.62±0.47.Table 3. FACT/GOG-Ntx Scale Scores in Patients Before Treatment (M±m)
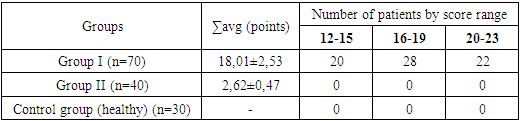 |
| |
|
For quantitative assessment of neuropathic pain in our study, we used the LANSS pain scale. According to the LANSS scale in the first group of patients before treatment, 11.4% were found to have a low probability of neuropathic pain mechanisms (<12 points), while 88.5% confirmed neuropathic pain mechanisms (>12 points). In the second group of patients, 100% of LANSS scale scores indicated the absence of neuropathic pain (<12 points).Table 4. LANSS Scale Scores in Patients Before Treatment (M±m)
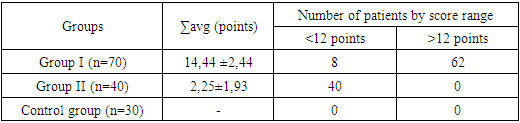 |
| |
|
4. Conclusions
The data indicate that CIPN manifests with various symptoms, including sensory, motor, and autonomic disturbances. The study identified specific patterns in the distribution of these symptoms: sensitivity reduction was most commonly observed in the lower extremities, while irritation symptoms were more evenly distributed between the upper and lower extremities. Approximately half of the patients experienced both loss of sensitivity and irritation symptoms simultaneously. These findings suggest complex mechanisms of CIPN development, which may be individual to each patient and dependent on the specific chemotherapy regimen. Overall, the data emphasize the diversity of clinical manifestations of CIPN and the importance of early detection and treatment to improve the quality of life for cancer patients undergoing chemotherapy.
References
| [1] | Hwang MS, Lee HY, Lee JH, et al. Protocol for a systematic review and meta-analysis of the efficacy of acupuncture and electroacupuncture against chemotherapy-induced peripheral neuropathy. Medicine (Baltimore). 2019; 98(14). doi:10.1097/MD.0000000000015098. |
| [2] | International Agency for Research on Cancer. (2020). GLOBOCAN 2020: Cancer Incidence and Mortality Worldwide. Retrieved from https://gco.iarc.fr/. |
| [3] | Jin Y, Wang Y, Zhang J, Xiao X, Zhang Q. Efficacy and safety of acupuncture against chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020; 2020: 8875433. doi:10.1155/2020/8875433. |
| [4] | Ledermann, J. A., Raja, F. A., Fotopoulou, C., Gonzalez-Martin, A., Colombo, N., & Sessa, C. (2013). Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 24 (suppl_6), vi24-vi32. |
| [5] | Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin. 2021; 71(3): 213-229. doi:10.3322/caac.21639. |
| [6] | Siegel, R. L., Miller, K. D., & Jemal, A. (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(1), 7-30. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


