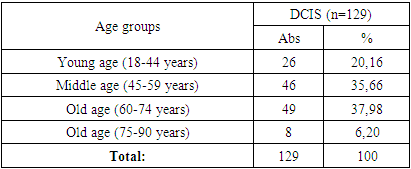Aziz Khakimov, Jamila Polatova
Tashkent State Dental Institute, Tashkent, Uzbekistan
Correspondence to: Aziz Khakimov, Tashkent State Dental Institute, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Breast cancer (BC) is one of the most common oncological pathologies in women. On average, BC is detected at the age of 61.5 years. The annual increase in the incidence of breast cancer is 2% in the new millennium, the cumulative risk is 5.87%, and the life expectancy of patients is 74 years. Modern diagnostic methods make it possible to detect breast cancer at stages I–II (71.2%), which has reduced mortality to 5.8% in the first year after detection, five-year survival reaches 60.9%, 65.5% of whom receive comprehensive treatment, and 34.5% only surgery. In the mid-20th century, DCIS (Ductal carcinoma in situ) was diagnosed extremely rarely and mainly with a significant volume of formation, reaching only 2% of new cases of breast cancer. The decrease in mortality in patients with DCIS is due to earlier detection of the oncological neoplasm, smaller tumor volume and lower incidence of the risk of a negative prognosis. The risk of DCIS is higher at the age of up to 50 years, in premenopause it stabilizes at 8-9% per year, and in menopause it decreases by 3% annually. Aim of the study: to study the results of surgical treatment of DCIS depending on the tumor size, degree of malignancy and immunohistochemical status based on the results of postoperative histological analysis. The study material included case histories of 129 women with DCIS aged 29-78 years, average age 56.44±11.92 years. Research methods: operational, histological and immunohistochemical with subsequent statistical processing of the obtained results. Introduction: in women with DCIS smaller than 15 mm, survival is statistically significantly better than with DCIS ≥15 mm (p≤0.05), in women with low or moderate nuclear differentiation of DCIS, survival is statistically significantly better than with high nuclear differentiation of DCIS (p≤0.05), i.e. the greatest concern for DCIS recurrence should be in young women with DCIS ≥15 mm in size with high nuclear differentiation.
Keywords:
DCIS, Survival, Immunohistochemistry, Histology
Cite this paper: Aziz Khakimov, Jamila Polatova, Histologic Features of DCIS and Their Impact on Survival, American Journal of Medicine and Medical Sciences, Vol. 14 No. 10, 2024, pp. 2472-2478. doi: 10.5923/j.ajmms.20241410.05.
1. Introduction
The identification of DCIS (Ductal carcinoma in situ) as a separate nosological entity in the structure of breast cancer (BC) occurred in the mid-20th century [11]. At that time, DCIS was diagnosed extremely rarely and mainly with a significant volume of formation, reaching only 2% of new cases of BC, and mastectomy was the gold standard for the treatment of such patients and often had good efficiency - it reduced the risk of recurrence below 1% within 5 years [14].DCIS diagnosed for the first time during screening has statistically significantly lower rates of invasive recurrence and mortality [7].The reduction in mortality is due to earlier detection of cancer, a smaller volume of DCIS and a lower incidence of the risk of a negative prognosis - a high degree of aggressiveness of tumor cells or comedonecrosis, which confirms the relevance of further study of early diagnostic markers of DCIS and the search for treatment methods comparable in effectiveness to mastectomy, since mastectomy reduces the risk of recurrence below 1% within 5 years [5].The risk of developing DCIS is higher before the age of 50, in premenopause it stabilizes at 8-9% per year, and in menopause it decreases by 3% annually [8,10].Early age at menarche is associated with an increased risk of premenopausal and postmenopausal DCIS, due to the rapid onset of regular ovulatory menstrual cycles and prolonged lifelong exposure to endogenous hormonal levels. [12] Early menarche also results in higher postmenopausal estrogen concentrations, which increases the risk of DCIS [4].Women who have not given birth have a higher risk of developing DCIS than women who have given birth, with the peak risk occurring in middle age – over 45 years old [15].But we should not forget about the negative consequences in terms of the risk of DCIS of the first pregnancy, especially a late one, after which an increase in the frequency of DCIS is noted in those who have given birth over several years relative to those who have not given birth at the same age [16].The risk of DCIS is characterized by an increase in the first 10 years after the first pregnancy [13]. This effect from subsequent pregnancies is much less pronounced, since the epithelium and alveoli of the mammary gland (MG) have already matured during the first gestation [6].Thus, the definition and clarification of the influence of clinical, anamnestic and radiation risk factors on the formation of DCIS recurrence and patient mortality is one of the most pressing research goals at the current stage of medical development.Aim of the study: to study the results of surgical treatment of DCIS depending on the tumor size, degree of malignancy and immunohistochemical status based on the results of postoperative histological analysis.
2. Material and Methods
The study material was the case histories of 129 women diagnosed with DCIS (TisN0M0) aged 29-78 years, average age 56.44±11.92 years, undergoing inpatient treatment and observation in the period from 2009 to 2017 in the surgical department of breast tumors at the Tashkent city branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology of Tashkent.The majority of the examined patients were elderly women – 49 (37.98%) and middle-aged women – 46 (35.66%). The distribution of the examined patients by age according to WHO criteria [1] is presented in Table 1.Table 1. Distribution of patients according to WHO age criteria
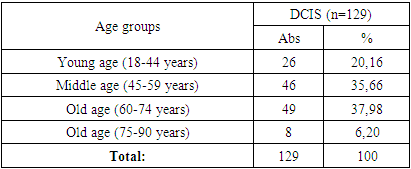 |
| |
|
Patients with a confirmed diagnosis of DCIS were divided into age groups, and according to the clinical TNM classification, they all corresponded to Tis (tumor in situ), i.e. the tumor was limited to the mammary gland duct without invasion into the duct walls, a tumor with no manifestation, without involvement of regional lymph nodes.The molecular subtype of DCIS was determined by immunohistochemical examination. The degree of tumor malignancy was assessed according to the Collage of American Pathologists scale: “Malignancy grade G1 – low; Malignancy grade G2 – intermediate; Malignancy grade G3 – high” [9]. In the study of postoperative material, an important role was played by the detection of DCIS with microinvasion and assessment of resection margins.The indicators of all studies were subjected to statistical analysis in the Microsoft Excel 2019 software package with the calculation of average values, standard errors of the mean, relative indicators, comparison of the obtained statistical characteristics with each other using the Student’s t-test [3]. Comparison of groups was performed using the Mann-Whitney criterion, the χ2 criterion and the Fisher criterion; the relationship between indicators was determined using the Spearman correlation coefficient and the Pearson method (r) [2].
3. Results and Discussion
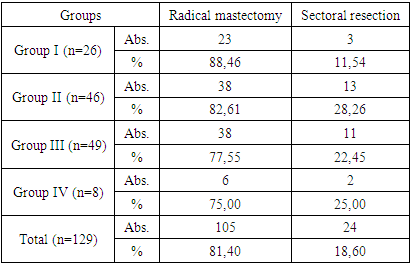
All patients studied underwent surgical treatment for DCIS, with radical mastectomy performed in 105 (81.4%) women and sectoral resection in 24 (18.60%).In the group of young women (Group I), radical mastectomy was performed in 23 (88.46%) women, sectoral resection – in 3 (11.54%), in Group II – 38 (82.61%), and 13 (28.26%), respectively, in Group III – 38 (77.55%) and 11 (22.45%), respectively, and in Group IV – 6 (75.0%) and 2 (25.0%), respectively.Table 2. Types of DCIS surgeries in the patients studied
 |
| |
|
After the surgical interventions, the obtained breast tumor tissues of all 129 patients were sent for histological examination. The average tumor volume during histological examination was 1.56±1.06 cm3 (0.2-9.0 cm3), the average tumor length was 14.38±7.74 mm, width – 11.82±7.56 mm, thickness – 8.04±3.15 mm. The presence of calcifications was found in 106 (82.17%) samples (Table 3).The resection margins were affected in 36 (27.91%) patients, while in the remaining 93 (72.09%) the resection margins was considered clean.In Group I, the resection margins were positive in 8 (30.77%) women, in Group II – in 12 (26.09%), in Group III – in 14 (28.57%), and in Group IV – in 25%. In all groups, the medial resection margin was predominantly positive.Re-excision with achievement of negative margins without resection was performed in 8 (6.2%) patients with an average length of 3.39±0.51 mm. Lobular cancerization was found in 11 (8.53%) patients.In our study, the average nuclear differentiation of DCIS was predominant – in 55 (42.64%) patients, low and high nuclear differentiation were distributed equally – in 37 (28.68%) patients each.According to the results of the histological examination, the average tumor length was 14.38±7.74 mm, the average width was 11.82±7.56 mm, the thickness was 8.04±3.15 mm, while there was no statistically significant difference between age groups.The average tumor volume was 1.56±1.06 cm3, also without statistically significant difference between the age groups of patients.During the histological examination, we meticulously determined the histological forms of DCIS.The most common and characteristic sign of low-differentiated DCIS was comedonecrosis - 71 (55.04%) tumors, without comedonecrosis - 15 (11.63%) tumors, in terms of groups, the maximum comedonecrosis was found in group IV - in 5 (62.50%), in group I - in 15 (57.69%), in group II - in 26 (56.52%) patients, the minimum - in group III - in 25 (51.02%) women (χ2=7,604, р=0,006).The morphologically common form was also cribriform – in 26 (20.16%) patients, solid DCIS – in 12 (9.30%) patients, the papillary form was found in 5 (3.88%) tumors.Table 3. Characteristics of DCIS histological examination by patient groups
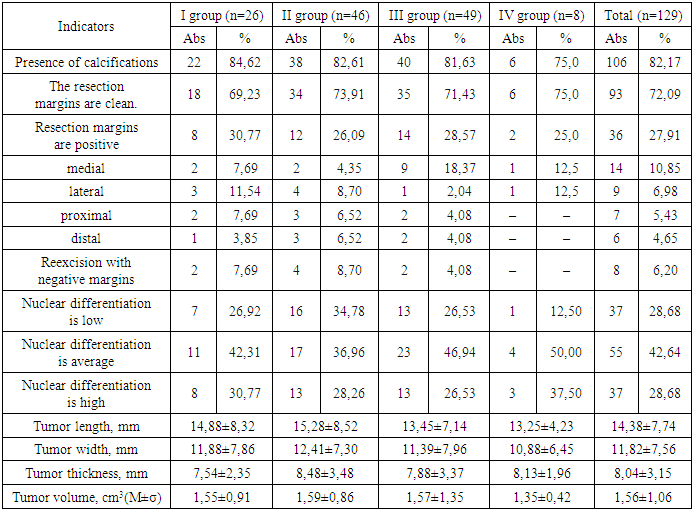 |
| |
|
The most common form of differentiation was the G1 form – highly differentiated tumor cells, slightly different from healthy ones with slow spread – in 53 (41.09%) patients, without statistical difference between the groups (p>0.05).The second most common type in the present study was G2 differentiation, characterized by the average malignancy of moderately differentiated carcinoma, in 47 (36.43%) women, and we found a statistically significant difference in the incidence of this form between groups I and IV (χ2=5.988, p=0.015), between groups II and IV (χ2=7.337, p=0.007), and other comparisons between groups did not reveal statistically significant differences (p>0.05).In our study, G3 differentiation was found in 29 (22.48%) breast tumors; comparative intergroup analysis did not reveal a statistically significant difference (р>0,05). The total histological assessment and DCIS grade score averaged 7.95±2.36; comparative intergroup analysis did not reveal a statistically significant difference (р>0,05).Based on the obtained results, we calculated and evaluated the Van Nuys prognostic index for each patient.Table 4. Histological forms and differentiation of DCIS by patient groups
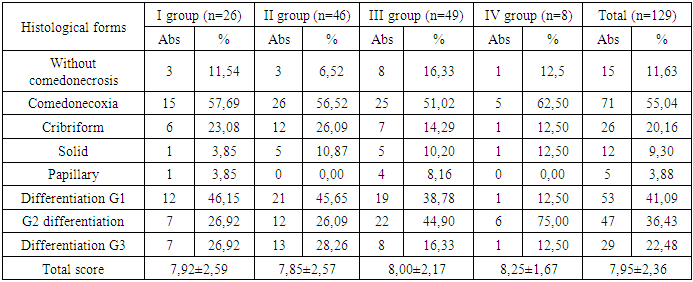 |
| |
|
There was no such indicator as the size of DCIS tumors ≥ 41 mm in our study, DCIS < 15 mm prevailed (89 (68.99%) patients) over DCIS 16-40 mm (40 (31.01%) patients) – (χ2=37.225, p<0.001), but comparative intergroup analysis did not reveal a statistically significant difference (p>0.05).In our study, the boundaries of normal tissue were less than 1 mm (93 (72.09%) patients), the boundaries of normal tissue of 10 mm and more were found in 29 (22.48%) patients, and normal tissue within 1-9 mm was found only in 7 (5.43%) patients. Comparative intergroup analysis did not reveal a statistically significant difference (p>0.05). Comparison of the difference in the occurrence of normal tissue had a statistically significant difference - thus less than 1 mm was statistically significantly more common than 10 mm and more - (χ2 = 63.691, p<0.001) and statistically significantly more common than 1-9 mm - (χ2 = 120.770, p<0.001).Among the DCIS we studied, tumors with G1/G2 differentiation without comedonecrosis predominated – 62 (48.06%), 38 (29.46%) were tumors with G1/G2 differentiation with comedonecrosis, and G3 differentiation with or without comedonecrosis – 29 (22.48%) tumors. It should be noted that comparative intergroup analysis did not reveal a statistically significant difference in each individual indicator (p>0.05).Comparison of the difference in the incidence of G1/G2 differentiation without comedonecrosis revealed a statistically significant predominance over G1/G2 differentiation with comedonecrosis – (χ2=9.406, p=0.003) and a statistically significant predominance over G3 differentiation with or without comedonecrosis – (χ2=18.488, p<0.001).On average, in our study, the majority – 62 (48.08%) – of patients were aged 40-60 years, 57 (44.19%) women over 60 years and only 10 (7.75%) under 40 years, which is statistically significant – (χ2=52.093, p<0.001) for 40-60 years and (χ2=44.536, p<0.001) for over 60 years of age relative to women under 40 years.The total VNPI score averaged 7.19±1.22 points with no intergroup difference (p>0.05).The performed trephine biopsy also demonstrates the absence of tumor differentiation in 7 (5.43%) patients and the predominance of G1 differentiation in 49 (37.98%) and G2 in 46 (35.66%) tumors, G3 differentiation was found in 27 (20.93%), which did not have a statistically significant difference in the average values and by groups (p>0.05).Among the breast tumors examined in this study, medium nuclear differentiation was predominant – 55 (42.46%), low and high nuclear differentiation – 37 (28.68%) tumors each; comparative intergroup analysis did not reveal a statistically significant difference (p>0.05).D3 level lymph node dissection, i.e. removal of the main (apical) RLUs at the base of the afferent artery [28,72‑79] was performed in 105 (81.40%) patients with DCIS, and minimal D0 lymph node dissection or its absence was performed in 24 (18.6%) patients. The average number of removed RLUs was 2.09±0.35 units, with a maximum of 3.13±0.68 units in group II and a minimum of 1.14±0.42 units in group III.When conducting immunohistochemical analyses, we found negative estrogen levels in 20 (15.5%) patients and positive levels in 109 (84.5%) of all subjects, negative progesterone levels in 24 (18.6%) patients and positive levels in 105 (81.4%) of all subjects.Table 5. Evaluation of the Van Nuys prognostic index for DCIS by patient groups
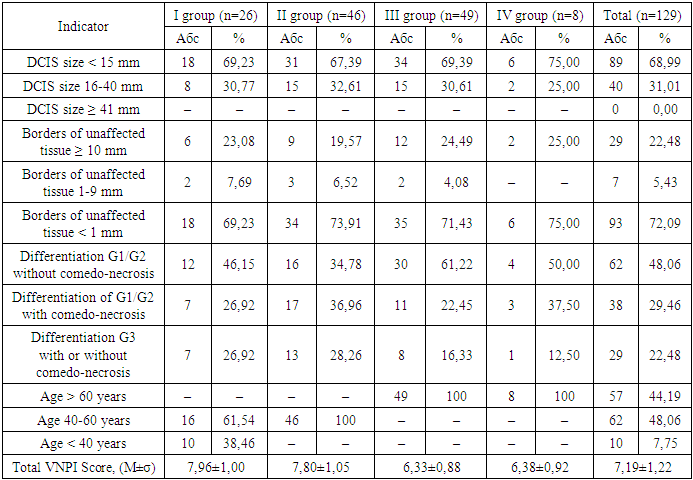 |
| |
|
Of all the patients studied, 109 (84.50%) received hormonal therapy from 0.5 to 5 years, its average duration was 3.98±1.81 years, in group I 22 (84.62%) patients received hormonal treatment for an average of 4.04±1.82 years, in group II - 40 (86.96%) patients with an average duration of 4.09±1.72 years, in group III - 42 (85.71%) patients with a duration of 4.04±1.77 years, in group IV - 5 (62.5%) with a duration of 2.88±2.47 years.All 129 patients studied received radiation therapy after the operations, 96 (74.42%) of them had no reaction to it, and 33 (25.58%) patients had a reaction to it, of which dermatitis - in 7 (21.21%), fatigue - in 22 (66.67%), breast swelling - in 1 (3.03%), chest pain - in 3 (9.09%) patients.Skin reaction in the form of epitheliitis of various stages was diagnosed in 7 (5.43%) patients - of which 6 (85.71%) had stage I and 1 (14.29%) had stage II. It should be taken into account that there were no statistically significant intergroup differences (p>0.05).In all 129 patients under study, after surgical, radiation and pharmacological treatment, we tracked the relapse and survival rates for 5 years. During the observation period, 82 (63.57%) survived 5 years, 47 (36.43%) did not survive 5 years, of the deceased patients, the average life expectancy was 3.72±1.12 years with a minimum of 1 year and a maximum of 4.5 years.In group I, 3 patients (11.54%) did not survive 5 years, their average life expectancy was 3.47±1.53 years (1-4.5 years), in group II - 8 women (17.39%), with an average life expectancy of 3.58±1.29 years (3-4.5 years), in group III, 15 (32.61%) died before the age of 5 with an average life expectancy of 3.40±1.53 years (1-4.5 years), in group IV - 4 (50%) died before the age of 5 with an average life expectancy of 3.45±1.54 years (1-4.5 years).Our analysis of survival depending on the size of DCIS, depending on the risk increase threshold we defined (up to 15 and ≥15 mm), allowed us to construct comparative survival curves for these groups of patients with DCIS (Fig. 1 and Table 6).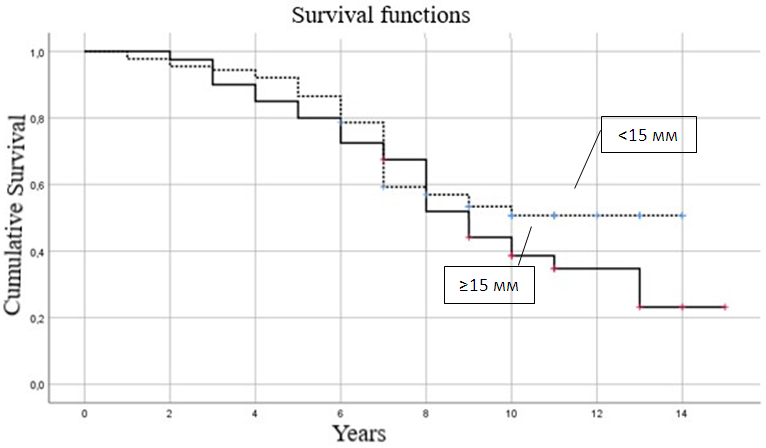 | Figure 1. Comparative analysis of survival curves depending on the size of DCIS |
Table 6. Survival rates by DCIS size, years
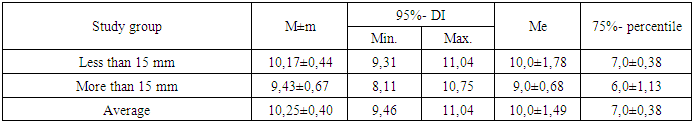 |
| |
|
Thus, of women with DCIS smaller than 15 mm, half will survive 10.0±1.78 years, 75% will survive the threshold of 7.0±0.38 years with an average life expectancy of 10.17±0.44 years (95% CI = 9.31-11.04 years), and with DCIS ≥15 mm, half will survive 9.0±0.68 years, 75% will survive the threshold of 6.0±1.13 years with an average life expectancy of 9.43±0.67 years (95% CI = 8.11-10.75 years). Thus, with DCIS smaller than 15 mm, a woman’s survival is statistically significantly better than with DCIS ≥15 mm (p≤0.05).We analyzed survival depending on the nuclear differentiation of DCIS depending on the risk increase threshold we determined (low + medium and high), which allowed us to construct comparative survival curves for these groups of patients with DCIS (Fig. 2 and Table 7).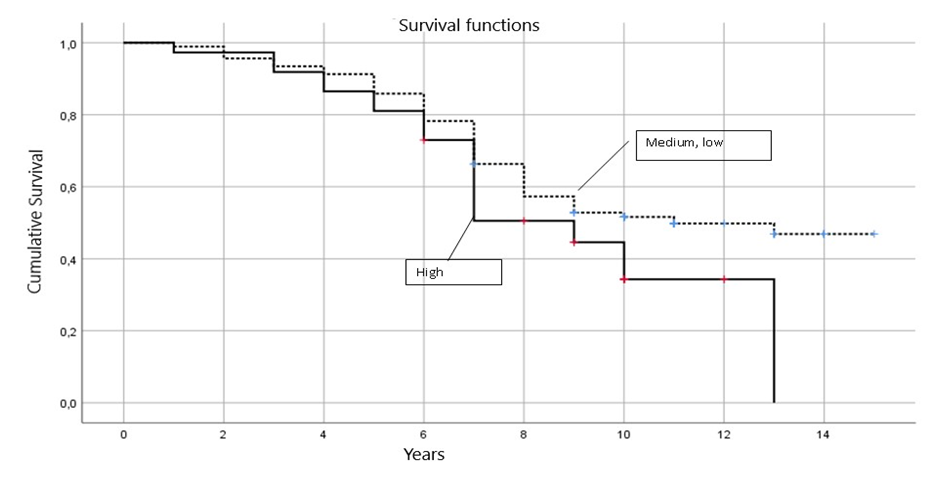 | Figure 2. Comparative analysis of survival curves depending on nuclear differentiation of DCIS |
Table 7. Survival rates depending on nuclear differentiation of DCIS, years
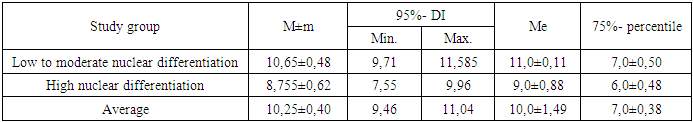 |
| |
|
Thus, of women with low or intermediate nuclear differentiation of DCIS, half will survive 11.0±0.11 years, 75% will survive the threshold of 7.0±0.50 years with an average life expectancy of 10.65±0.48 years (95% CI = 9.71-11.585 years), and with high nuclear differentiation of DCIS, half will survive 9.0±0.88 years, 75% will survive the threshold of 6.0±0.48 years with an average life expectancy of 8.755±0.62 years (95% CI = 7.55-9.96 years). Thus, with low or intermediate nuclear differentiation of DCIS in women, survival is statistically significantly better than with high nuclear differentiation of DCIS (p≤0.05).The survival data presented indicate the need for early diagnosis (to detect minimal tumor size) and screening programs, especially among young women (under 44 years of age).
4. Conclusions
As follows from the presented data, with increasing age the clinical condition of patients worsens, but the role of immunohistochemical processes of the type in such patients is important, which determine more severe prognoses along with the risks according to the Van Nuys assessment for DCIS.Comparison of the difference in the incidence of G1/G2 differentiation without comedonecrosis revealed a statistically significant predominance over G1/G2 differentiation with comedonecrosis – (χ2=9.406, p=0.003) and a statistically significant predominance over G3 differentiation with or without comedonecrosis – (χ2=18.488, p<0.001).On average, in our study, the majority – 62 (48.08%) – of patients were aged 40-60 years, 57 (44.19%) women over 60 years old and only 10 (7.75%) under 40 years old, which is statistically significant – (χ2=52.093, p<0.001) for 40-60 years old and (χ2=44.536, p<0.001) for over 60 years old relative to women under 40 years old.In all 129 patients under study, after surgical, radiation and pharmacological treatment, we tracked the relapse and survival rates for 5 years. During the observation period, 82 (63.57%) survived 5 years, 47 (36.43%) did not survive 5 years, of the deceased patients, the average life expectancy was 3.72±1.12 years with a minimum of 1 year and a maximum of 4.5 years.In group I, 3 patients (11.54%) did not survive 5 years, their average life expectancy was 3.47±1.53 years (1-4.5 years), in group II – 8 women (17.39%), with an average life expectancy of 3.58±1.29 years (3-4.5 years), in group III, 15 (32.61%) died before the age of 5 with an average life expectancy of 3.40±1.53 years (1-4.5 years), in group IV – 4 (50%) died before the age of 5 with an average life expectancy of 3.45±1.54 years (1-4.5 years).The average survival of patients with DCIS was 10.25±0.4 years (95% CI=9.46-11.04), with the median survival time being 10.0±1.49 years, the 75% percentile being 7.0±0.38 years. Of women with DCIS over 45 years of age, half will survive 10.0±2.11 years, 75% will survive past the threshold of 7.0±0.41 years with an average survival time of 10.53±0.45 years (95% CI=9.66-11.40 years), and of patients with DCIS under 44 years of age, half will survive past the threshold of 8.0±1.79 years, 75% will survive past the threshold of 7.0±1.26 years with an average survival time of 8.75±0.80 years (95% CI= 7.18-10.315 years); of women with low or intermediate nuclear differentiation of DCIS, half will survive 11.0±0.11 years, 75% will survive the threshold of 7.0±0.50 years with an average life expectancy of 10.65±0.48 years (95% CI= 9.71-11.585 years), and with high nuclear differentiation of DCIS, half will survive 9.0±0.88 years, 75% will survive the threshold of 6.0±0.48 years with an average life expectancy of 8.755±0.62 years (95% CI= 7.55-9.96 years).Conclusion. In women with DCIS <15 mm in size, survival is statistically significantly better than with DCIS ≥15 mm in size (p≤0.05), in women with low or moderate nuclear differentiation of DCIS, survival is statistically significantly better than with high nuclear differentiation of DCIS (p≤0.05), i.e. the highest alertness for DCIS recurrence should be caused by young women with DCIS ≥15 mm in size with high nuclear differentiation.
5. Recommendations
Include a detailed comparative analysis between different surgical methods and their impact on survival and recurrence rates among various age groups. Expand the methodology section by providing more details about the immunohistochemical techniques used, specifying the antibodies and staining procedures. Use multivariate analysis to assess the impact of multiple factors such as age, tumor size, and nuclear differentiation on patient outcomes. Highlight the implications of the findings for clinical practice, particularly in determining the suitability of conservative surgery versus mastectomy for different patient profiles. Include a more detailed breakdown of patient demographics, such as menopausal status, family history, and other comorbidities, to provide better context for the study results. Discuss the limitations of the study, such as sample size and retrospective nature, and suggest areas for future research to explore further correlations or refine diagnostic criteria. Strengthen the conclusion by not only summarizing the findings but also suggesting actionable steps for improving early diagnosis and personalized treatment strategies for DCIS. Consider adding more graphs or figures to visually represent the statistical data, making the information more accessible and easier to interpret.
References
| [1] | Classification of ages adopted by the World Health Organization (WHO) // http://www.who.int/topics/classification/ru/ https://agesecrets.ru/vozrast/vozrastnaya-klassifikatsiya-vsemirnoj-organizatsii-zdravoohraneniya#i. |
| [2] | Medik V.A., Tokmachev M.S., Fishman B.B. Statistics in medicine and biology: a manual in 2 volumes. T.1. Theoretical statistics. - M.: Medicine, 2000. - 412 p. |
| [3] | Rebrova O.Yu. Statistical analysis of medical data. Application of the STATISTICA application package - M.: Media Sphere, 2006. - 3rd ed. - 312 p. |
| [4] | Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. // Biol Res. 2017 - №50(1) – р.33-41. |
| [5] | Allred DC, Wu Y, Mao S, Nagtegaal ID, Lee S, Perou CM, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. // Clin Cancer Res 2018 - №14 – р.370–378. |
| [6] | Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. // Updates Surg. 2017 - №69(3) – р.313-317. |
| [7] | Cheung S, Booth ME, Kearins O, Dodwell D. Risk of subsequent invasive breast cancer after a diagnosis of ductal carcinoma in situ (DCIS). // Breast, 2014, - №23 – р.807–811. |
| [8] | Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. // Am J Epidemiol. 2020 - №152(10) – р.950-64. |
| [9] | Collage of American Pathologists. Protocol for the Examination of Resection Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast. Version: 4.4.0.0. June 2021. p.17. |
| [10] | Katsura C, Ogunmwonyi I, Kankam HK, Saha S. Breast cancer: presentation, investigation and management. // Br J Hosp Med (Lond). 2022 - №83(2) – р.1-7. |
| [11] | Khan MM, Yalamarty SSK, Rajmalani BA, Filipczak N, Torchilin VP. Recent strategies to overcome breast cancer resistance. // Crit Rev Oncol Hematol. 2024 - №197 – р.104-111. |
| [12] | Kolak A, Kamińska M, Sygit K, Budny A, Surdyka D, Kukiełka-Budny B, Burdan F. Primary and secondary prevention of breast cancer. Ann Agric Environ Med. 2017 - №24(4) – р.549-553. |
| [13] | Li Z, Wei H, Li S, Wu P, Mao X. The Role of Progesterone Receptors in Breast Cancer. // Drug Des Devel Ther. 2022 - №16 – р. 305-314. |
| [14] | Schnitt SJ, Silen W, Sadowsky NL, Connolly JL, Harris JR. Ductal carcinoma in situ (intraductal carcinoma) of the breast. // N Engl J Med. 2018 - №318(14) – р.898–903. |
| [15] | Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. // Lancet. 2015 - №365(9472) – р.1727-41. |
| [16] | Zhang YN, Xia KR, Li CY, Wei BL, Zhang B. Review of Breast Cancer Pathologigcal Image Processing. // Biomed Res Int. 2021 - №221 – р.199-204. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML