-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2422-2428
doi:10.5923/j.ajmms.20241409.64
Received: Aug. 23, 2024; Accepted: Sep. 19, 2024; Published: Sep. 30, 2024

Study of Properties of Polyvalent Serum of Intestinal Yersinosis Obtained from Experimental Animals Using Different Immunization Schemes
Tadjiyeva N. U.1, 2, Kosimov O. Sh.1, 3, Abdullaev A. O.4, Anvarov J. A.1, 2, Mirzoeva M. R.5, Dauletbaev A. D.4
1Republican Specialized Scientific-Practical Medical Center of Epidemiology, Microbiology, Infectious and Parasitic Diseases, Tashkent, Uzbekistan
2Tashkent Medical Academy, Tashkent, Uzbekistan
3Tashkent Research Institute of Vaccines and Serums, Uzbekistan
4Kimyo International University in Tashkent, Uzbekistan
5Bukhara State Medical Institute, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article describes the results of obtaining polyvalent serum in experimental animals by hyperimmunization, as well as the dynamics of total protein, albumin, globulins, IgA, IgM, IgG indicators, serum activity using extended agglutination reaction in slides and tubes at the stages of hyperimmunization. An increase in the level of globulins was observed as an immune response in experimental animals on days 7-14-21-28 of immunization. In blood sera obtained from experimental animals co-injected with inactivated corpuscular and soluble antigens of serovar strains of Yersinia enterocolitica, high levels of total protein, albumin, globulin and IgG were observed on the 28th day of the experiment.
Keywords: Intestinal yersiniosis, Yersinia enterocolitica, Hyperimmunization, Polyvalent serum, Total protein, Albumin, Globulin, IgA, IgM, IgG, Agglutination, Antigen
Cite this paper: Tadjiyeva N. U., Kosimov O. Sh., Abdullaev A. O., Anvarov J. A., Mirzoeva M. R., Dauletbaev A. D., Study of Properties of Polyvalent Serum of Intestinal Yersinosis Obtained from Experimental Animals Using Different Immunization Schemes, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2422-2428. doi: 10.5923/j.ajmms.20241409.64.
1. Introduction
- Currently, bacterial infections are a global problem. An example among such bacteria that cause dangerous infections is Yersinia. Yersinia is a bacterium from the family Enterobacteriaceae, gram-negative bacilli, facultative anaerobes. It includes 18 species, the most common of which are: Yersinia pseudotuberculosis, Yersinia enterocolitica and Yersinia pestis (causative agent of plague) [1,2,6,10]. Enteropathogenic Yersinia include the causative agents of pseudotuberculosis and intestinal yersiniosis. The causative agent of intestinal yersiniosis is Yersinia enterocolitica. The main reservoir of the pathogen is rodents [3,4].The bacteriological method used in the diagnosis of intestinal yersiniosis requires high labor intensity, long periods of pathogen isolation, low efficiency, gives a large number of false isolations of yersiniosis cultures, which is associated with significant contamination of pathological material with intestinal microflora and imperfection of isolation methods. Serologic diagnostic methods significantly increase the efficiency of Yersinia isolation [5,7].Standard sera are important for the efficiency of serologic testing. Standard sera are obtained by hyperimmunization. Hyperimmunization is a method of parenteral administration of increasing doses of the corresponding antigens to animals in order to obtain the highest immunological response of the organism and, consequently, the maximum increase of specific antibodies in the blood of animals, which should provide therapeutic, prophylactic and diagnostic effect of preparations [8,11].In order to improve measures against intestinal yersiniosis, it is necessary, first of all, to improve methods of diagnostics of this infection. Agglutinating sera should be obtained to detect the causative agents of the main serovars (Yersinia enterocolitica O5, Yersinia enterocolitica O9) obtained from patients and the external environment. This, in turn, creates the basis for early and timely diagnosis of intestinal yersiniosis and appropriate preventive measures.The purpose of the study is to investigate the properties of polyvalent sera of intestinal yersiniosis obtained from experimental animals under different immunization schemes.For hyperimmunization 12 rabbits weighing from 2.3 to 3.3 kg at the age of 4 to 6 months were used. The experiments were conducted in accordance with the methodological manual [9] approved by the Ministry of Health of the Republic of Uzbekistan in 2016 “Methods and rules for working with laboratory animals in experimental microbiological and immunological studies”.
2. Bacteriologic Method
- Serovars O3 Yersinia enterocolitica 005011/659 and O9 Yersinia enterocolitica 005008/656 were used for serum production. Experimental animals were injected with a suspension of cultures of these strains prepared at various concentrations according to the McFarland standard and cultured on neutral agar. The experimental animals were quarantined for 21 days.The rabbits were divided into 4 groups. 3 rabbits were taken into each group:- in group I at the 1st immunization rabbits were inoculated with Yersinia enterocolitica 005011/659 strain of serovar O3 corpuscular microbial cells with a concentration of 4 billion cl/mL, at the 2nd 8 billion cl/mL, at the 3rd 16 billion cl/mL, at the 4th 20 billion cl/mL and at the 5th 25 billion cl/mL,- in group II at the 1st immunization was inoculated with Yersinia enterocolitica 005011/659 strain of serovar O3 with a concentration of 4 billion cl/mL of soluble and corpuscular antigen in a ratio of 1:1, at the 2nd 4 billion cl/mL of soluble antigen, at the 3rd 16 billion cl/mL of soluble and corpuscular antigen. cl/mL of soluble and corpuscular antigen in a 1:1 ratio, at the 4th 20 billion cl/mL of soluble and corpuscular antigen in a 1:1 ratio, and at the 5th 25 billion cl/mL of soluble antigen, - in group III at the 1st immunization was inoculated with Yersinia enterocolitica 005008/656 strain O:9 of corpuscular microbial cells with a concentration of 4 billion cl/mL, at the 2nd 8 billion cl/mL, at the 3rd 16 billion cl/mL, at the 4th 20 billion cl/mL and at the 5th 25 billion cl/mL,- in group IV at the 1st immunization was inoculated with Yersinia enterocolitica 005008/656 strain of serovar O:9 with a concentration of 4 billion cl/mL of soluble and corpuscular antigen in a ratio of 1:1, at the 2nd 4 billion cl/mL of soluble antigen, at the 3rd 16 billion cl/mL of soluble and corpuscular antigen. cl/mL of soluble and corpuscular antigen in a 1:1 ratio, at the 4th 20 billion cl/mL of soluble and corpuscular antigen in a 1:1 ratio, and at the 5th 25 billion cl/mL of soluble antigen..Each of 12 rabbits in each of the 4 groups was injected with 1.6 ml of inactivated Yersinia enterocolitica culture or soluble antigen at 8 points: the first 4 points were injected subcutaneously along the spine on both sides (1-4), the next 4 points were injected intramuscularly into the muscles of both front and hind legs (5-8). The volume in each point was 0.2 ml.Serological method: Conducted on the basis of the order of the Ministry of Health of the Republic of Uzbekistan № 170 from April 19, 2004 “On improvement of measures to combat yersiniosis”.Immunologic studies. A reagent kit (Vector Best, Russian Federation) was used for immune-enzymatic determination of total immunoglobulins A, M and G, as well as immunoglobulins of classes M and G to intestinal Yersinia pathogens. The results were evaluated according to the manufacturer's instructions.The amount of total protein, albumin and globulin was analyzed by enzymatic-colorimetric method on the biochemical analyzer “Mindray” VA-88A - medical equipment of the Chinese company. The results were evaluated according to the manufacturer's instructions.Statistical method. The digital material was processed by the method of variation statistics using the program “Excel-Office” 2013 with the use of Student's t-criterion. The mean square error (m) was calculated, as well as the reliability of differences between the values in the compared groups. Differences were considered reliable at p<0.05. Nominal data were described with absolute values and percentages. Nominal data were compared using Pearson's χ2 test, Fisher's exact test. Differences were considered reliable at p<0.05.
3. Results and Discussion
- Changes in the amount of albumin and globulins in the composition of total protein and protein fractions are considered a response of the organism to hyperimmunization. Globulins are involved in the transport of lipids, hormones, vitamins and metal ions in the body, and their importance in the immune system has been proven. An increase in the level of globulins as an immune response was observed in the body of experimental animals on days 7, 14, 21 and 28 of immunization (Table 1). The increasing trend of total protein content on 7, 14, 21 and 28 days of immunization also indicates the activation of defense mechanism and albumin content increased from 34.03±1.126 g/L to 54.30±0.794 g/L due to the increase in total protein content raised to 70.33±2.345 g/L.
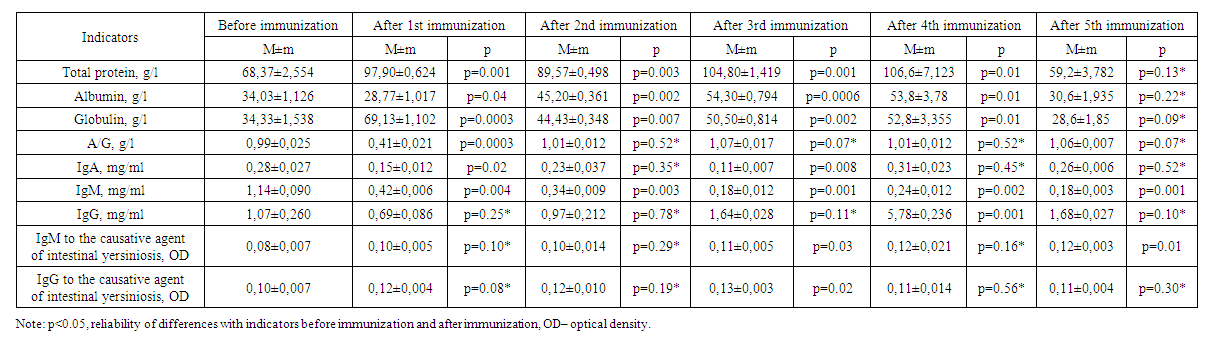 | Table 1. Results of immunization of experimental animals of the first group |
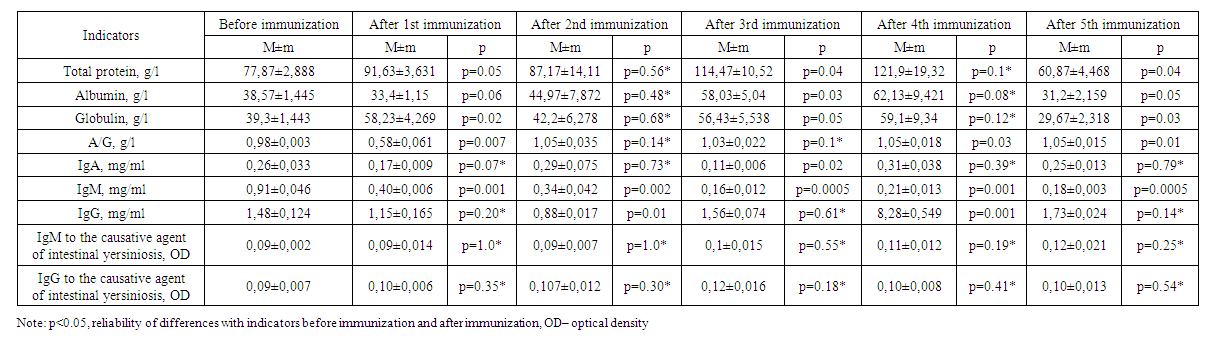 | Table 2. Results of immunization of experimental animals of the second group |
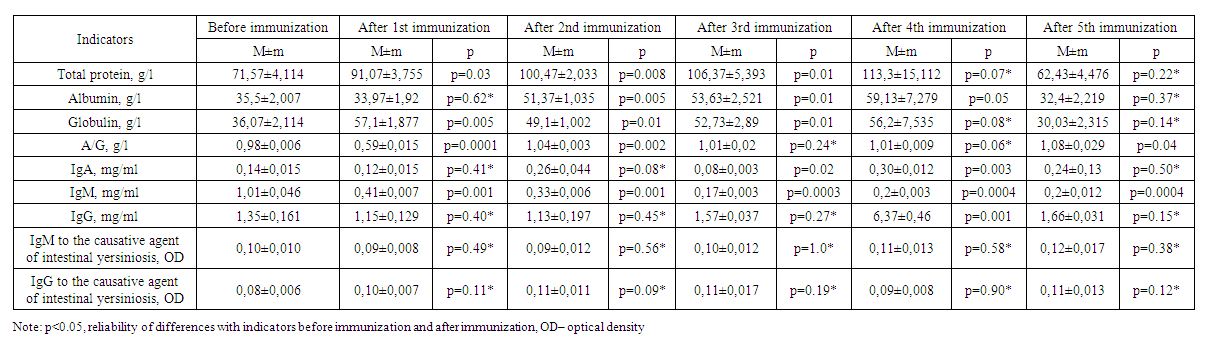 | Table 3. Results of immunization of experimental animals of the third group |
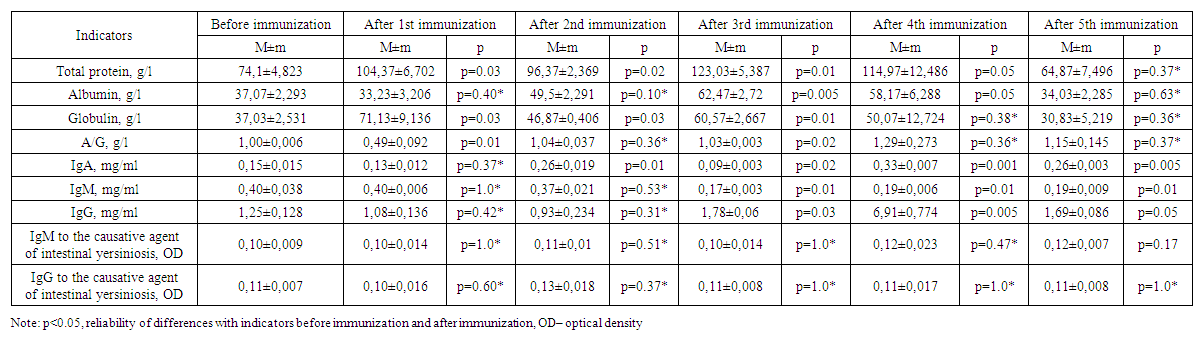 | Table 4. Results of immunization of experimental animals of the fourth group |
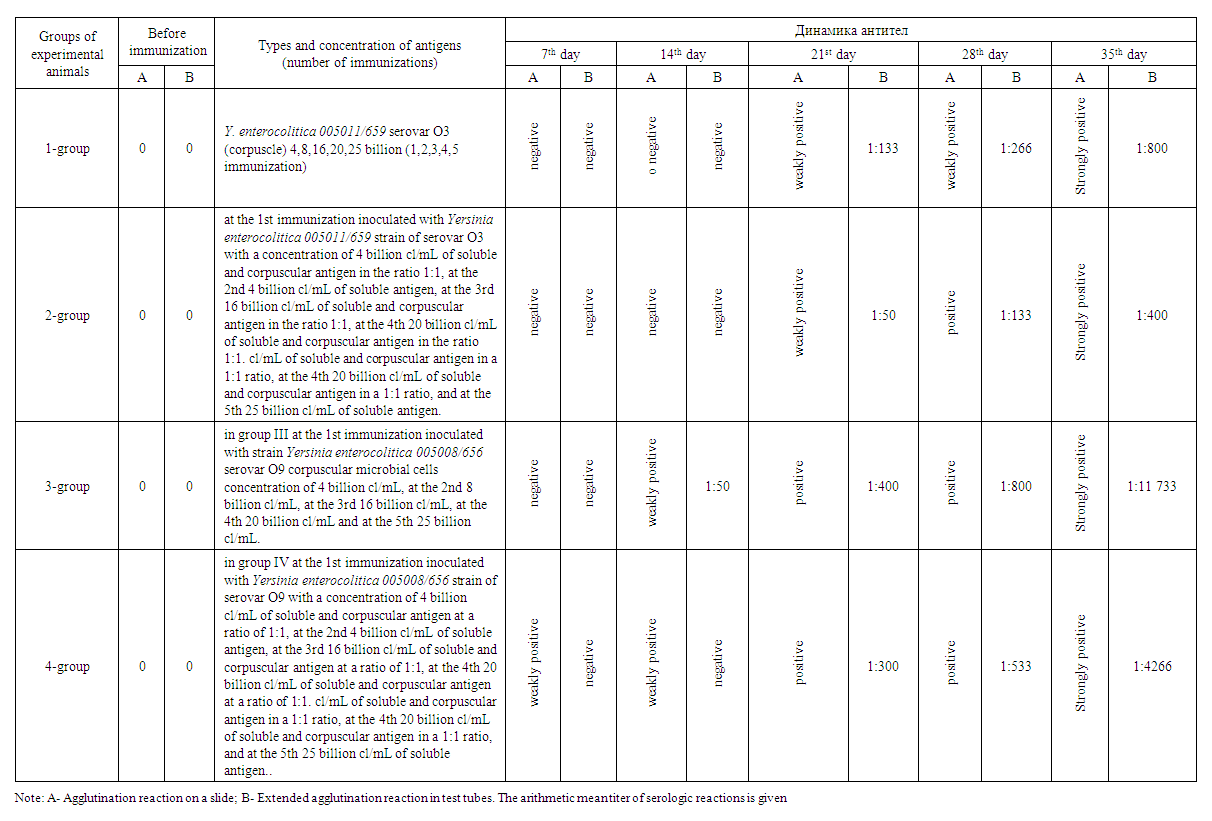 | Table 5. Dynamics of antibody titer changes in experimental animals immunized with Yersinia enterocolitica strains |
4. Conclusions
- 1. Experimental animals showed an increase in globulins as an immune response on days 7-, 14-, 21- and 28 of immunization.2. On the 28th day of the experiment, high levels of total protein, albumin, globulin, and IgG were detected in the sera of group 2 animals that were co-injected with corpuscular and soluble antigens of Yersinia enterocolitica serovar O3 strains.3. In serum, immunoglobulin A increased on the 14th-28th day, decreased on the 21st-35th day, immunoglobulin M decreased on the 14th day, decreased on the 21st day, and a stable trend was observed after 28-35 days. 4. No significant changes of IgM and IgG indices in relation to the pathogen of intestinal yersiniosis were revealed.5. On the 35th day of immunization, i.e. on the fifth immunization with antigens, all indicators gave sharply positive results.6. After the third immunization, i.e. from the 21st day of immunization, a dynamic increase in antibody titer was observed in all groups when blood sera obtained from experimental animals were examined by in vitro agglutination reaction.7. Considering that the values of total protein, albumin, globulin and IgG in experimental rabbits are highest on the 28th day of immunization, sera with high titers can be obtained from the animals after the 4th week.8. To obtain a full-fledged active hyperimmune serum it is necessary to use inactivated corpuscular and soluble antigens together.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML