-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2411-2415
doi:10.5923/j.ajmms.20241409.61
Received: Aug. 27, 2024; Accepted: Sep. 22, 2024; Published: Sep. 30, 2024

Age Microanatomical Characteristics of Bends (Muscle Jam) of the Duodenum of Rats
Ilyasov Aziz Saidmuratovich1, Turaev Fazliddin Sadriddinovich2, Nazarova Feruza Normamatovna3
1Doctor of Biological Sciences, Associate Professor, Navаi Innovation University, Uzbekistan
2Bukhara Regional Medical Association, Uzbekistan
3Navаi Innovation University, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

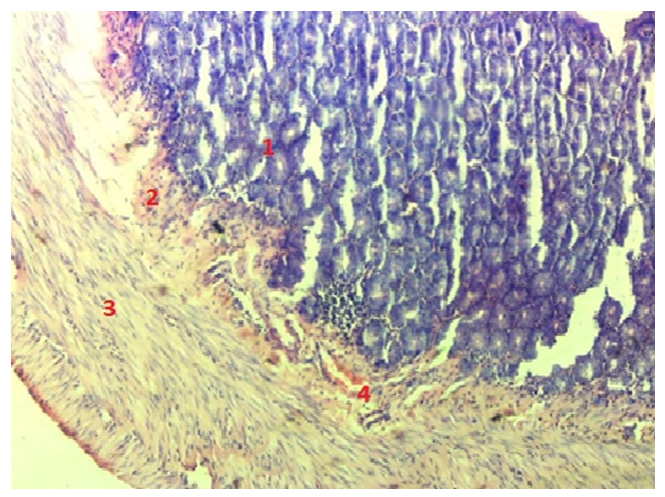
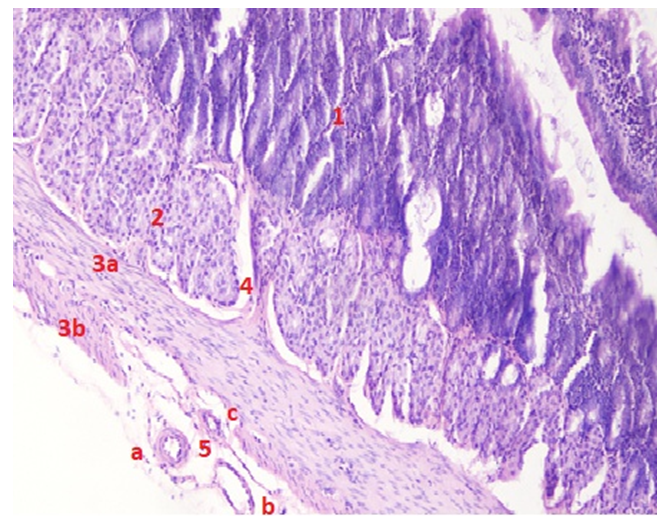
When studying the duodenal wall histologically, we found that the muscle layer and its collagen fibers in the area of bends are thicker than in the areas before and after the bend. Also, when comparing the bends themselves, it turned out that the muscle layer is thickest in the lower bend, and thinnest in the subhepatic bend. The connective tissue fibers along the wall of the duodenum are located uniquely in the muscle layer in the area of the bends. In the intestine, bundles of collagen fibers are located parallel to the intestine in the submucosal layer, and the bundle located near the muscular plate changes its direction and is directed towards the lamina propria of the mucous layer. Forms loops of different sizes. The last bundles of fibers form the basal part of the single-layer epithelium.
Keywords: Duodenum, Sphincter, Muscle jam, Collagen fibers, Flexure, Circular muscle, Longitudinal muscle
Cite this paper: Ilyasov Aziz Saidmuratovich, Turaev Fazliddin Sadriddinovich, Nazarova Feruza Normamatovna, Age Microanatomical Characteristics of Bends (Muscle Jam) of the Duodenum of Rats, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2411-2415. doi: 10.5923/j.ajmms.20241409.61.
Article Outline
1. Relevance of the Topic
- Recently, a number of experimental studies have appeared that significantly expand the general idea of the role and importance of the structure of the intestinal wall and sphincters. It is known that sphincter apparatus in the digestive canal are functionally active zones that are very important in regulating the passage of food and chymus. Due to the presence of sphincters, the entire digestive tract is divided into separate cavities (oral cavity, stomach) or parts of one organ (intestine), which are characterized by their participation in the mechanism of maintaining the stability of the characteristic osmotic pressure, intra-cavity pressure, microflora and environment. The sphincter is a specially formed muscle tissue (smooth or transverse) that allows you to control the volume and/or duration of contact between the divisions (segments, parts) of the hollow organ. Sphincters, which are simple in our understanding, are actually a special part of the closing apparatus, consisting of the following sections: the part before the sphincter, the sphincter itself, and the part after the sphincter [4,10].L.L. Kolesnikov In his works, emphasizes the current and promising direction in "Sphincterology" - biology and medicine, which justifies the study of the complex structure and function of the apparatus, such as the sphincter. All this raises the problem of studying the sphincters of the digestive system to a new level. This direction is very promising and of great importance for Theoretical and applied medicine. All sphincters of the digestive tract allow moving the food Mass in one direction and prevent their reflux (reverse movement). The cause of the development of many non-infectious diseases of the digestive system is a violation of the function of the sphincter apparatus [2,15]. Therefore, the interest of many scientists in studying the morphology and pathology of the sphincter apparatus does not decrease. The sphincters of the digestive tract play a leading role in regulating the movement of food mass, and are the focus not only of morphologists, physiologists, but also of pathologists, clinical doctors, since sphincters represent sites of localization of inflammatory processes, ulcers and tumors.Morfologlarning ta'rifiga ko`ra: sfinkter - bu har qanday naysimon organning jomi, yopiluvchi qismi mushak qatlamining halqasimon mushaklarining qalinlashishi [1,15]. Gistologik tuzilishga ko`ra, barcha sfinkterlar ko`ndalang targ`il - rabdosfinkterlar va silliq mushakli - leyosfinkterlarga bo`lingan [3,7]. L.L. Kolesnikov (2008) studied in detail the direction of the muscles in the pores. He noted that, as a rule, there are no separate contractions of the longitudinal and annular muscles. F.F. According to Sax (1994) often in the digestive system, when part of the longitudinal muscle fibers pass straight over the sphincter, more through the puncture of the sphincter annular muscles. These and other fibers intersect spirally with one. Therefore, their interaction ensures not only contraction, but also the opening and closing of the sphincter socket. The simplest sign of the sphincter is the narrowed area of the organ cavity and the presence of thickened annular muscles [10]. Compared to the longitudinal axis of the organ, the location of the sphincter is often oblique than transverse. The presence of an angle between the two adjacent organs and the presence of a cavity in a serrated form is an important quality of the antireflux mechanism [5,13]. The uniformity of the thickness of the sphincter circumference wall is mainly at the expense of the floor of the annular muscles. For example, in the area of the pyloric sphincter, the muscle floor is sufficiently thickened along the large and small curvature of the stomach. In the ileocecal sphincter, longitudinal muscle fibers, spirally twisted, penetrate between the annular muscle fibers and form a backbone with rhombic cells. In general, the spiral and transverse fibers provide expansion of the organ cavity. The mucous membrane of the sphincter area is 15-28% thicker than in other areas. [14]. The coordinated tonic activity of these sphincters ensures their functional function, which makes it possible to group them into the sphincter apparatus [8,10].The longitudinal layer of the muscular layer of the human rectum is distributed along the circumference of the rectum and descends downwards to the upper edge of the inner sphincter of the anal canal. Here, the diameter of the rectum becomes significantly smaller. The muscle layers that cover it from the outside tend to converge sharply and sometimes overlap. Nearby layers exchange for muscle-elastic elements. Ilyasov A.S. (2021) and Yukhimets S.N. (2010), Lar believes that with one side they touch the outer sphincter layers, with the other side touching the outer surface of the inner sphincter of the anal canal. A large number of elastic threads are directed from the surface of the pads towards the inner sphincter. Elastic filaments pass through the sphincter to the surface of the intestinal cavity. Here, the muscular bases of the anal columns are fixed. Elastic filaments from the bottom of the pads cover the lower edge of the inner sphincter and rise from the bottom up to the same anal columns [8,9]. Ilyasov A.S.according to (2021), the length of the anal canal of the rectum averages 1625.6±35.3 µm. During Postnatal ontogenesis, a greater increase in anal canal length was recorded during the 11 to 16 days of development of bats, which is associated with the transition from breast feeding to mixed feeding. The length of the internal sphincter in newborn rats is 720.7 ± 25.0 µm. The length of the internal sphincter varies in the process of structural formation. The greatest growth rates of internal sphincter length are determined at ages 6 and 11 days. By the age of 3 months, the rate of growth of the length of the internal sphincter decreases by 2 times compared to the previous age. This may be due to its functional development. Bag devices provide relative anatomical and functional autonomy of the digestive tract and thus maintain the constancy of the environment in them [5].The sphincter apparatus has its own auxiliary elements-folds of the mucous membrane, veins under the mucous membrane. The subcutaneous veins are located above the sphincters, forming bulky venous tangles that act as characteristic "cushions" when opening and closing the sphincter [8]. But the muscular elements of the sphincters are not distributed in the same way everywhere. For example, the pyloric or anal sphincters are well developed, but the Cardial, bulbo-duodenal sphincters are worse developed. Above and below each sphincter, at a certain distance, receptors of the mucous membrane of the digestive tract accumulate, forming reflexogenic zones. Exposure of the reflexogenic zone under the sphincter increases its tone, preventing reflux (reverse direction). [6,12]. The structural support of the sphincter device is currently decomposed into macroscopic, and microscopic structures. This should be taken into account when performing surgical, radiological or endoscopic examinations [11]. In the literature, we can also find the term "sphincter system" [15], which includes muscles, myoepithelial elements and auxiliary sphincter mechanisms. In the scientific literature, information is sufficient about the micro anatomical structure of the digestive canal muscular jacks (sphincters) and how they are arranged in relation to connective tissue fibers, but, as a result of the specific structure of the muscles in the area of folds of the duodenum and the interaction of these folds with connective tissue fibers, the relationship of each.Thus, in the field of transition of one organ to the second organ in the organs of the hive, information about sphincters (caps, muscular Joms) is fully reflected, but, in the scientific literature, the anatomical functional features of the muscular Joms and especially the duodenum in the area of the duodenum and its folds are not fully illuminated.
2. The Purpose of the Work
- To study the age-related microanatomic structures of squamous (muscular jaws) in the intestinal wall of rats twelve fingers.
3. Materials and Research Methods
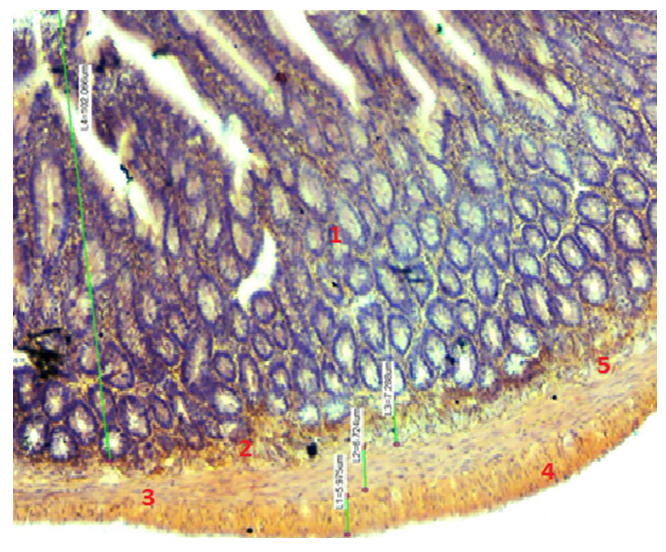
- The study was conducted under standard vivarian conditions in 41 different age groups, including 13 at 3 months, 14 at 6 months, and 14 at 12 months in white-breed rats. For a week before the start of the study, sexually mature rats were observed in quarantine conditions. No somatic or infectious disease was transferred to normal vivarian conditions after observation.All groups were formed at the same time. Laboratory animals involved in the experiment were selected by age, weight, living and feeding conditions.The experiment of the appropriate duration was carried out by slaughtering animals, the usual decapitation under the influence of ether narcosis in the morning on an empty stomach. Twelve fingers for morphological and morphometric study of the structure of the intestinal wall twelve fingers are the subcutaneous fold of the intestine, the upper fold, the lower fold and 12 b.i. - a comparative morphological analysis and morphometry was performed by taking a lump from the folds of the hungry intestine.Tissue samples were hardened with 10% formalin for 24 hours. Then, after rinsing under running water for 3 hours, the step by step was dehydrated with graded series of ethanol (70-80-96-100%), purified in a mixture of xylol and xylol-paraffin, and then released into paraffin produced in Russia by LLC "BIOVITRUM".Paraffin-embedded tissue Microtome (Reichert - Jung 2040, Leica Corp., Watzlar, Germany) were successively divided into 3 µm thick cross sections and installed in the glass windows of the item (Citotest Labvare Manufacturing Co., Ltd. Standard Chinese level item glass). The incisions purified from paraffin are van - Gizone-stained, with hematoxylin and eosin (GE). After staining, the incisions were successively dehydrated in ethanol, purified in xylol, and the coating was glazed.The incisions were morphometrically examined using the DN-107t / Model NLCD-307b (Roman, China) ocular micrometer, measuring the thickness of the mucous membrane of the duodenum, subcutaneous, muscle and serous membranes, informative parts of the incision were photographed. General morphological changes in the studied parts of the duodenum were studied by staining the hematoxylin-eosin and Van Gizon method.
4. Results
- Results of individual examination: in rats of different ages, the duodenum consists of three: the upper, descending, ascending part and four: the subcutaneous, upper, lower, twelve finger - hungry intestinal folds, which differ from the morphological and morphometric hexate in that these areas improve with age change during postnatal ontogenesis. The thickness of the duodenal wall during the three - month - reproductive period of rats is equal to the average in the area of the subcutaneous tract-652.7±14.79 µm. It becomes slightly thinner in the areas up to the bend and after the bend. The thickness of the inner annular floor is on average 133.6±3.03 µm in the flexion area, and this muscle contraction ensures mass mixing in the intestinal cavity. The outer longitudinal muscle thickness averages 63.1±1.43 µm. During the three - month period of rats, the thickness of the duodenal wall is on average-725.3±6.09 µm in the area of high flexion.
5. Conclusions
- the muscles of the muscle connective tissue fibers play an important role in the formation of Joms in the flexion area of the duodenum. At the same time twelve fingers form a functional unit that separates one part of the intestine from the other. The part of the collagen fiber Tufts in the mucosal layer on the muscle floor, pointing along the circular muscle floor, forms clumps of various sizes that surround the circular muscle cells in the area of the flexion.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML